Elements Of Group 17 Are Called
Muz Play
Mar 20, 2025 · 6 min read
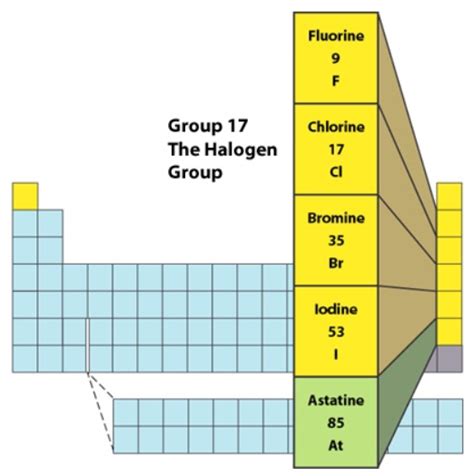
Table of Contents
Elements of Group 17 are Called Halogens: A Deep Dive into Their Properties, Reactions, and Applications
The elements of Group 17, also known as halogens, are a fascinating family of nonmetals that exhibit a unique set of properties and play crucial roles in various applications. Understanding their characteristics, reactions, and uses is fundamental to appreciating their significance in chemistry and beyond. This comprehensive article delves deep into the world of halogens, exploring their individual properties, chemical behaviors, and widespread applications.
What are Halogens?
Halogens are located in Group 17 (or VIIA) of the periodic table. The name "halogen" originates from Greek words meaning "salt-former," reflecting their tendency to react with metals to form salts. These elements include fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and astatine (At). Tennessine (Ts), a synthetically produced element, is also considered a halogen, but its properties are less well-understood due to its extreme radioactivity and short half-life.
Key Properties of Halogens
Halogens share several key characteristics:
1. Electronic Configuration and Reactivity
Halogens have seven valence electrons in their outermost shell, giving them a strong tendency to gain one electron to achieve a stable octet configuration. This high electronegativity makes them highly reactive, especially with alkali metals and alkaline earth metals. Their reactivity generally decreases down the group, with fluorine being the most reactive and astatine the least.
2. Physical States and Colors
At standard temperature and pressure, halogens exhibit varying physical states:
- Fluorine (F): Pale yellow gas
- Chlorine (Cl): Greenish-yellow gas
- Bromine (Br): Reddish-brown liquid (the only non-metallic liquid element at room temperature)
- Iodine (I): Dark grey-black solid that sublimes (transitions directly from solid to gas) readily
- Astatine (At): Black solid; highly radioactive and exists only in trace amounts
The colors deepen as you go down the group, reflecting changes in their electronic structure and interactions with light.
3. Oxidation States
Halogens typically exhibit a -1 oxidation state in their compounds, reflecting their tendency to gain one electron. However, they can also exhibit positive oxidation states, particularly in compounds with more electronegative elements like oxygen and fluorine. For example, chlorine can exist in oxidation states of +1, +3, +5, and +7.
4. Diatomic Molecules
Halogens exist as diatomic molecules (X₂) in their elemental form, meaning two atoms of the same halogen are bonded together. This is due to the strong covalent bonds formed between the two atoms to achieve a stable electron configuration. For example, chlorine gas exists as Cl₂.
5. Boiling and Melting Points
The boiling and melting points of halogens increase down the group. This is due to increasing intermolecular forces (specifically van der Waals forces) as the size of the halogen atoms increases and the number of electrons increases.
Chemical Reactions of Halogens
Halogens undergo a wide range of chemical reactions, primarily due to their high reactivity:
1. Reactions with Metals
Halogens readily react with metals to form ionic compounds called halides. These reactions often involve vigorous exothermic processes (releasing heat). For instance, the reaction between sodium and chlorine produces sodium chloride (table salt):
2Na(s) + Cl₂(g) → 2NaCl(s)
2. Reactions with Nonmetals
Halogens also react with several nonmetals, forming covalent compounds. For example, chlorine reacts with hydrogen to form hydrogen chloride (HCl), a highly corrosive gas:
H₂(g) + Cl₂(g) → 2HCl(g)
3. Displacement Reactions
Halogens can displace less reactive halogens from their compounds. This is because a more reactive halogen will oxidize a less reactive halogen. For example, chlorine can displace bromine from potassium bromide:
Cl₂(g) + 2KBr(aq) → 2KCl(aq) + Br₂(l)
This trend follows the reactivity series, with fluorine being the most reactive and astatine the least.
Applications of Halogens
Halogens and their compounds have a vast array of applications in various industries:
1. Fluorine
- Teflon (Polytetrafluoroethylene): A non-stick coating used in cookware, and in various industrial applications due to its high thermal stability and chemical inertness.
- Refrigerants: Certain fluorocarbons were used as refrigerants, although their contribution to ozone depletion led to the development of hydrofluorocarbons (HFCs) as safer alternatives. However, HFCs are also potent greenhouse gases and are increasingly being phased out.
- Dentistry: Fluoride compounds are used in toothpastes and dental treatments to strengthen tooth enamel and prevent cavities.
2. Chlorine
- Water Purification: Chlorine is widely used as a disinfectant in water treatment plants and swimming pools to kill harmful bacteria and viruses.
- Bleaching: Chlorine-based compounds are used as bleaching agents in the paper and textile industries.
- PVC (Polyvinyl Chloride): A versatile plastic used in pipes, flooring, and other applications.
- Solvents: Chlorinated solvents were once commonly used, but many have been phased out due to environmental concerns.
3. Bromine
- Flame Retardants: Brominated flame retardants are used in plastics, textiles, and electronics to prevent fires. However, environmental concerns regarding their persistence and potential toxicity are leading to their gradual phase-out.
- Agricultural Chemicals: Bromine compounds are used as fumigants and pesticides.
- Photography: Silver bromide is used in photographic film.
4. Iodine
- Medicine: Iodine is an essential trace element in the human body and is used as a disinfectant and antiseptic. Iodine deficiency can lead to various health problems.
- Nutrition: Iodized salt is used to prevent iodine deficiency disorders.
- Dyeing: Iodine compounds are used as dyes.
5. Astatine
Astatine's highly radioactive nature severely limits its practical applications. Its extremely short half-life means it exists only in trace quantities. Research focuses primarily on understanding its chemical properties and potential applications in nuclear medicine.
Environmental Concerns and Safety Precautions
While halogens have numerous beneficial applications, some pose significant environmental and health concerns:
- Ozone Depletion: Chlorofluorocarbons (CFCs) and halons have been linked to ozone depletion in the stratosphere. International agreements like the Montreal Protocol have successfully reduced their production and use.
- Global Warming: Certain halogenated compounds, including HFCs, are potent greenhouse gases that contribute to climate change.
- Toxicity: Many halogenated compounds are toxic to humans and the environment. Careful handling and disposal are crucial.
- Persistence: Some halogenated compounds are persistent organic pollutants (POPs), meaning they resist degradation and can accumulate in the environment and living organisms.
Conclusion: The Enduring Importance of Halogens
The elements of Group 17, the halogens, represent a crucial and diverse group of elements. Their high reactivity, varied physical properties, and diverse applications make them essential in numerous industrial processes, technological advancements, and biological functions. However, careful consideration must be given to the potential environmental and health implications associated with certain halogenated compounds to ensure their responsible and sustainable use. Ongoing research continues to explore new applications while also mitigating the negative consequences of some halogens and their compounds. Understanding the properties and behavior of halogens is critical for developing sustainable solutions for the future.
Latest Posts
Latest Posts
-
Lineweaver Burk Plot Of Competitive Inhibition
Mar 21, 2025
-
What Kind Of Change Forms A New Substance
Mar 21, 2025
-
1 2 Cos Alpha As A Product
Mar 21, 2025
-
Compound Interest And Simple Interest Worksheet
Mar 21, 2025
-
What Is A Sanction In Sociology
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Elements Of Group 17 Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
