Elements That Are Gaseous At Room Temperature
Muz Play
Mar 25, 2025 · 7 min read
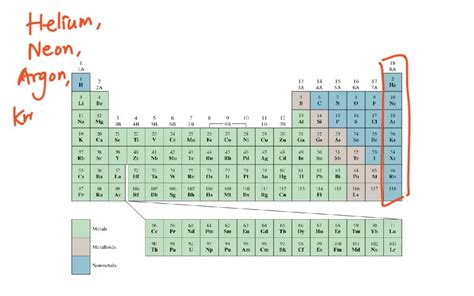
Table of Contents
Elements That Are Gaseous at Room Temperature: A Deep Dive
The periodic table is a vast landscape of elements, each with unique properties and behaviors. Among these, a select few exist as gases at standard room temperature (around 25°C or 77°F and 1 atm pressure). Understanding these gaseous elements is crucial in various fields, from industrial applications to atmospheric science and even biological processes. This comprehensive guide explores these fascinating elements, delving into their properties, uses, and significance.
The Noble Gases: A Family of Unreactive Gases
The most well-known gaseous elements are the noble gases, also known as inert gases. Located in Group 18 of the periodic table, these elements are characterized by their exceptional stability and minimal reactivity. This inertness stems from their complete electron shells, leaving them with little tendency to gain, lose, or share electrons to form chemical bonds.
Helium (He)
- Atomic Number: 2
- Properties: Colorless, odorless, tasteless, and non-toxic. It's the lightest noble gas and has the lowest boiling point of any element, making it incredibly difficult to liquefy. Helium is also significantly less dense than air.
- Uses: Helium finds widespread applications, particularly in cryogenics (cooling systems for MRI machines and superconducting magnets), as a lifting gas in balloons and airships (safer than hydrogen), and as a protective atmosphere in welding and other industrial processes. Its low reactivity makes it ideal for these applications. It's also used in leak detection and as a carrier gas in gas chromatography.
- Abundance and Sources: Helium is relatively abundant in the universe, but its scarcity on Earth makes it a valuable resource. It's extracted primarily from natural gas deposits.
Neon (Ne)
- Atomic Number: 10
- Properties: Neon is also colorless, odorless, and tasteless. Its most striking characteristic is its brilliant reddish-orange glow when an electric current passes through it, making it ideal for signage.
- Uses: Neon lighting is its most iconic use. It is also employed in lasers, high-voltage indicators, and some types of vacuum tubes.
- Abundance and Sources: Neon is extracted from air via fractional distillation. Although present in trace amounts in the atmosphere, its extraction is commercially viable.
Argon (Ar)
- Atomic Number: 18
- Properties: Argon is an inert, colorless, odorless, and tasteless gas. It's significantly more abundant in the atmosphere than neon.
- Uses: Argon's primary use is as an inert atmosphere in industrial processes like welding and metal production, protecting the molten metal from oxidation. It's also used in incandescent light bulbs to prevent filament oxidation and in lasers.
- Abundance and Sources: Argon is the third most abundant gas in the Earth's atmosphere, making it readily available from air separation plants.
Krypton (Kr)
- Atomic Number: 36
- Properties: Krypton is a colorless, odorless, and tasteless noble gas. It glows with a pale whitish-yellow light when electrically excited.
- Uses: Krypton is used in some high-intensity lighting applications, such as high-powered flash lamps in photography and specialized lasers. It is also found in some fluorescent lights to improve their efficiency and color rendition.
- Abundance and Sources: Krypton is extracted from the atmosphere during air liquefaction and separation. It is present in much smaller concentrations than Argon.
Xenon (Xe)
- Atomic Number: 54
- Properties: Xenon is a colorless, odorless, and tasteless noble gas. It emits a bluish light when electrically stimulated.
- Uses: Xenon is employed in high-intensity arc lamps used in projectors and automotive headlamps. It's also used in some medical applications, including anesthesia and as a contrast agent in medical imaging. Xenon-based lasers are used in various applications, including eye surgery.
- Abundance and Sources: Xenon is extracted from air during the liquefaction and fractionation process. It is even less abundant than Krypton.
Radon (Rn)
- Atomic Number: 86
- Properties: Radon is a radioactive, colorless, odorless, and tasteless gas. Its radioactivity makes it hazardous to human health.
- Uses: Despite its radioactivity, radon has limited applications, primarily in radiation therapy and geological research.
- Abundance and Sources: Radon is a decay product of radium, found in certain rocks and soils. Its presence in homes is a significant health concern due to its radioactivity.
Beyond the Noble Gases: Other Gaseous Elements
While the noble gases are the most well-known group of gaseous elements, several others also exist in gaseous form at room temperature.
Hydrogen (H₂)
- Atomic Number: 1
- Properties: Hydrogen is the lightest element, colorless, odorless, and tasteless under normal conditions. It is highly flammable and reactive.
- Uses: Hydrogen is used extensively in ammonia production (Haber-Bosch process), as a fuel (both currently and in the context of future hydrogen economies), in the refining of petroleum, and in various chemical processes.
- Abundance and Sources: Although the most abundant element in the universe, hydrogen is not found freely in the Earth's atmosphere in large quantities. It's produced industrially from natural gas and other hydrocarbon sources.
Nitrogen (N₂)
- Atomic Number: 7
- Properties: Nitrogen is a colorless, odorless, and tasteless gas, and constitutes about 78% of Earth's atmosphere. It's relatively unreactive at room temperature.
- Uses: Nitrogen is used extensively in the production of ammonia (fertilizers), as an inert atmosphere in food packaging to prevent oxidation, and in the manufacturing of various chemicals. Liquid nitrogen is used as a coolant.
- Abundance and Sources: Nitrogen is readily available from the atmosphere through fractional distillation.
Oxygen (O₂)
- Atomic Number: 8
- Properties: Oxygen is a colorless, odorless, and tasteless gas that is essential for respiration in most living organisms. It's highly reactive and supports combustion.
- Uses: Oxygen has countless applications, most significantly in respiration, various industrial processes (e.g., steel production, welding), and medical applications (e.g., respiratory support).
Abundance and Sources: Oxygen is the second most abundant gas in the Earth's atmosphere and is readily obtained through fractional distillation of air.
Fluorine (F₂)
- Atomic Number: 9
- Properties: Fluorine is a pale yellow, highly reactive and toxic gas. It's the most electronegative element, meaning it readily attracts electrons in chemical bonds.
- Uses: Fluorine is used in the production of various fluorinated compounds, including refrigerants, plastics (like Teflon), and pharmaceuticals. It's also used in uranium enrichment.
- Abundance and Sources: Fluorine is not found in its elemental form in nature due to its high reactivity. It's obtained through the electrolysis of its compounds.
Chlorine (Cl₂)
- Atomic Number: 17
- Properties: Chlorine is a greenish-yellow gas with a pungent odor. It's highly toxic and reactive.
- Uses: Chlorine is used in water purification (disinfection), in the production of various chemicals (e.g., plastics, solvents), and as a bleaching agent.
- Abundance and Sources: Chlorine is not found freely in nature but is obtained through the electrolysis of sodium chloride (salt).
Bromine (Br₂)
- Atomic Number: 35
- Properties: Bromine is a reddish-brown liquid at room temperature, but it readily evaporates to form a reddish-brown gas with a pungent odor. It's toxic and corrosive.
- Uses: Bromine is used in the production of flame retardants, pesticides, and certain dyes.
- Abundance and Sources: Bromine is extracted from seawater and brine deposits.
Environmental Significance and Safety Considerations
The gaseous elements play crucial roles in the environment and human health. Oxygen's essentiality for respiration is paramount, while nitrogen's abundance in the atmosphere is vital for the nitrogen cycle. However, some gaseous elements pose significant environmental and health risks. For instance, excessive greenhouse gas emissions (including some resulting from industrial processes using gaseous elements) contribute to climate change. Furthermore, the toxicity and reactivity of elements like chlorine and fluorine require strict safety measures in handling and storage. Radon's radioactivity is a significant health concern, necessitating monitoring and mitigation efforts in homes and buildings.
Conclusion: A Diverse and Essential Group
The gaseous elements, encompassing noble gases, reactive diatomic gases and more, represent a diverse group with wide-ranging applications and implications. Their properties, from the inertness of noble gases to the reactivity of halogens, determine their uses in various industries, scientific research, and environmental processes. Understanding their characteristics and handling them safely is essential for harnessing their benefits while mitigating potential risks. Further research into their applications and environmental impacts will continue to shape various technologies and our understanding of the natural world.
Latest Posts
Latest Posts
-
The Martian And The Car Answer Key
Mar 27, 2025
-
Rusting Of Iron Chemical Or Physical Change
Mar 27, 2025
-
How Many Neutrons Does K Have
Mar 27, 2025
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Elements That Are Gaseous At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
