A Larger Nucleus Splits Apart Making 2 Smaller Ones
Muz Play
Mar 27, 2025 · 6 min read
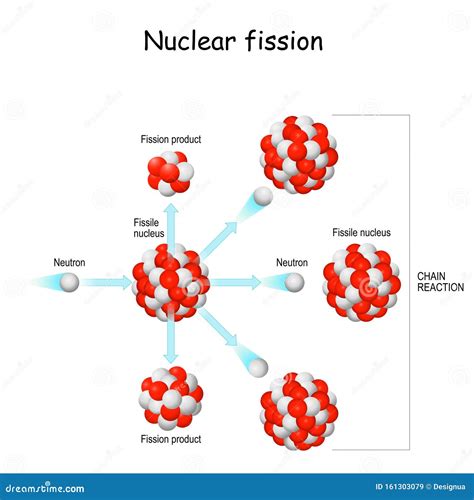
Table of Contents
Nuclear Fission: When a Larger Nucleus Splits Apart
Nuclear fission, the process where a large atomic nucleus splits into two or more smaller nuclei, is a fundamental concept in nuclear physics with far-reaching implications for energy production, weaponry, and scientific research. Understanding this process requires delving into the forces governing the nucleus, the energy released, and the diverse applications and implications of fission.
The Structure of the Atomic Nucleus
Before exploring fission, let's briefly revisit the atomic nucleus. At the heart of every atom lies the nucleus, a dense region containing protons and neutrons, collectively known as nucleons. Protons carry a positive charge, while neutrons are electrically neutral. The strong nuclear force, an incredibly powerful but short-range interaction, binds these nucleons together, overcoming the electrostatic repulsion between the positively charged protons. The stability of a nucleus depends on the delicate balance between the strong nuclear force and the electromagnetic repulsion.
Isotopes and Nuclear Stability
Atoms of the same element can have different numbers of neutrons, resulting in isotopes. While all isotopes of an element share the same number of protons (defining their atomic number), they can have varying numbers of neutrons (affecting their atomic mass). Some isotopes are stable, meaning their nuclei remain intact indefinitely. Others are unstable or radioactive, undergoing nuclear decay to achieve a more stable configuration. This instability often manifests as radioactive decay, a process that involves emitting particles or energy to transform into a more stable isotope.
The Mechanics of Nuclear Fission
Nuclear fission occurs when a heavy, unstable nucleus, typically one with a high atomic number, absorbs a neutron. This absorption triggers an imbalance in the nuclear forces, leading to the nucleus becoming highly unstable and prone to splitting. The nucleus doesn't simply break apart randomly; the process is governed by the complex interplay of nuclear forces and the quantum mechanical nature of the nucleus.
The Role of the Neutron
The neutron plays a crucial role as an initiator in fission. Because it's electrically neutral, it can easily penetrate the nucleus without being repelled by the positive charge of the protons. Once inside, the neutron adds to the overall mass and energy of the nucleus, pushing it beyond its limit of stability. This instability triggers the fission process.
Fission Products and Energy Release
When the nucleus splits, it doesn't break into two equal halves. Instead, the fission products – the smaller nuclei formed – are usually of unequal size. This uneven splitting is due to the complex forces at play within the nucleus. The fission products are typically radioactive isotopes of lighter elements, often emitting beta particles (electrons) and gamma rays as they transition towards greater stability.
The most striking aspect of fission is the massive release of energy. This energy release stems from the difference in the binding energy per nucleon between the parent nucleus and the fission products. The binding energy is the energy required to hold the nucleons together. Heavier nuclei have a lower binding energy per nucleon compared to lighter nuclei, meaning they are less tightly bound. When a heavy nucleus fissions, the resulting lighter nuclei are more tightly bound, releasing the excess energy as kinetic energy of the fission products, gamma radiation, and neutrons. This energy is enormous, far exceeding the energy released in chemical reactions.
Types of Nuclear Fission
Not all fission events are the same. Different isotopes exhibit varying fission characteristics, leading to different outcomes. The number of neutrons released, the energy released, and the types of fission products vary depending on the parent nucleus and the energy of the incoming neutron.
Spontaneous Fission
While most fission events are induced by neutron absorption, some heavy nuclei can undergo spontaneous fission. In this case, the nucleus spontaneously splits without any external intervention. Spontaneous fission is a rare event, but it plays a role in determining the radioactivity of certain isotopes.
Induced Fission
Induced fission, as described earlier, is triggered by the absorption of a neutron. This is the most commonly studied and utilized type of fission, as it forms the basis of nuclear reactors and weapons.
The Chain Reaction
The released neutrons from a fission event are crucial for sustaining a chain reaction. These neutrons can then be absorbed by other fissile nuclei, triggering further fission events. If the conditions are right – enough fissile material is present, and the neutrons are not escaping – the process can become self-sustaining, leading to a rapid increase in energy release.
Critical Mass
The concept of critical mass is vital for understanding chain reactions. The critical mass is the minimum amount of fissile material required to sustain a chain reaction. If the mass is below critical, too many neutrons escape without causing further fission, and the reaction will die out. If the mass exceeds critical, the chain reaction will escalate rapidly, potentially leading to an explosion, as in a nuclear weapon.
Applications of Nuclear Fission
The immense energy released during fission has led to its application in various fields:
Nuclear Power Generation
Nuclear power plants utilize controlled fission chain reactions to generate electricity. Fissile material, usually uranium-235 or plutonium-239, is used as fuel. The heat generated by the fission process is used to boil water, creating steam that drives turbines connected to electric generators. This provides a powerful and relatively low-carbon source of energy, though with associated risks related to nuclear waste and safety.
Nuclear Weapons
The uncontrolled chain reaction in a nuclear weapon leads to a massive, almost instantaneous release of energy, resulting in a devastating explosion. The destructive power of nuclear weapons stems from the rapid release of a huge amount of energy, causing immense blast damage, thermal radiation, and radioactive fallout.
Scientific Research
Nuclear fission has played a crucial role in scientific research, providing insights into the structure of the nucleus, nuclear forces, and the behavior of matter at extreme energies. Research involving fission has furthered our understanding of fundamental physics and has been instrumental in various scientific advancements.
Medical Applications
While less directly related to fission itself, the radioactive isotopes produced during the fission process have found applications in medicine, such as in medical imaging and radiotherapy.
Safety and Environmental Concerns
The use of nuclear fission is not without its challenges:
Nuclear Waste
The fission process generates radioactive waste, posing a significant long-term environmental challenge. This waste remains radioactive for thousands of years, requiring safe and secure storage solutions. Managing and disposing of nuclear waste is a complex and ongoing issue.
Nuclear Accidents
The potential for accidents at nuclear facilities, such as the Chernobyl and Fukushima disasters, highlights the need for robust safety measures and regulatory oversight to prevent catastrophic events.
Nuclear Proliferation
The potential for the misuse of nuclear fission technology for weapons production raises concerns about nuclear proliferation and international security.
Conclusion
Nuclear fission is a powerful process with transformative potential. Its ability to release vast amounts of energy has revolutionized energy production and has had significant impacts on various scientific and technological fields. However, the inherent risks associated with fission, including nuclear waste, accidents, and proliferation, necessitate careful management and responsible utilization to maximize its benefits while mitigating its potential negative consequences. Ongoing research and technological advancements are crucial for improving the safety and sustainability of nuclear fission technology and ensuring its responsible application for the benefit of humanity. The future of nuclear energy likely hinges on addressing the challenges surrounding waste management, safety regulations, and the development of safer and more efficient reactor designs. A deeper understanding of the process, its applications, and the challenges it presents remains essential for informed decision-making in this crucial area of science and technology.
Latest Posts
Latest Posts
-
How To Find Moles From Volume
Mar 30, 2025
-
Sig Fig Multiplication And Division Practice
Mar 30, 2025
-
Describe How Mass And Inertia Are Related
Mar 30, 2025
-
Density Of Water 22 Degrees Celsius
Mar 30, 2025
-
What Does The Nernst Equation Tell Us
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about A Larger Nucleus Splits Apart Making 2 Smaller Ones . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
