Elements That Are Gases At Room Temperature
Muz Play
Mar 31, 2025 · 6 min read
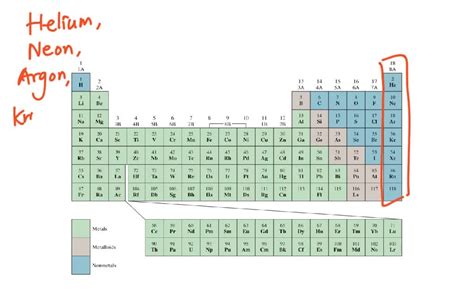
Table of Contents
Elements That Are Gases at Room Temperature: A Deep Dive
The periodic table is a treasure trove of information, showcasing the diverse properties of elements that make up our world. Among these properties, the state of matter at room temperature is a fundamental characteristic that influences an element's behavior and applications. This article delves into the fascinating world of elements that exist as gases at room temperature, exploring their properties, uses, and environmental impact. Understanding these elements is crucial across various scientific disciplines, from chemistry and physics to environmental science and engineering.
The Noble Gases: The Unreactive Giants
The most well-known group of gaseous elements at room temperature are the noble gases. Located in Group 18 of the periodic table, these elements—helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn)—are characterized by their exceptional stability and inertness. Their outermost electron shell is completely filled, meaning they have little tendency to gain, lose, or share electrons with other atoms. This full valence shell makes them exceptionally unreactive, hence their designation as "noble."
Helium (He): The Lighter Than Air Wonder
Helium, the second lightest element, is famously known for its low density, making it less dense than air. This property leads to its extensive use in balloons and blimps. Beyond recreational applications, helium's inertness and low reactivity are invaluable in various scientific and industrial processes. It's used as a cryogenic refrigerant, enabling low-temperature research and applications like MRI machines. Helium's unique properties also make it crucial in leak detection, welding, and as a shielding gas in arc welding processes.
Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn): Diverse Applications
Neon, argon, krypton, xenon, and radon, while less common in everyday life than helium, have diverse applications. Neon's characteristic bright red-orange glow is widely recognized in neon signs, while argon's inertness makes it an essential shielding gas in welding and metal production. Krypton finds use in high-intensity lighting, such as in some types of flash photography. Xenon, with its unique spectral lines, has applications in high-intensity lamps and as a general anesthetic. Radon, however, is radioactive and presents a significant health hazard due to its potential to cause lung cancer. Its presence in buildings is a major concern for public health.
Beyond the Nobles: Other Gaseous Elements
While the noble gases are the most prominent examples, other elements also exist as gases at room temperature. These elements display varying degrees of reactivity and have diverse applications.
Hydrogen (H): The Abundant and Reactive Element
Hydrogen, the simplest and most abundant element in the universe, is a highly reactive gas at room temperature. It readily combines with other elements, forming a vast array of compounds. Its most notable compound is water (H₂O). Hydrogen's reactivity makes it a promising fuel source, as its combustion with oxygen produces only water, making it a relatively clean energy source. However, challenges remain in its safe storage and transportation. It's also used in the production of ammonia (NH₃), a crucial component of fertilizers. Hydrogen is a vital element in many industrial processes, demonstrating its diverse role in the modern economy.
Nitrogen (N): The Atmospheric Abundant Element
Nitrogen, a major component of Earth's atmosphere (approximately 78%), is a relatively unreactive gas at room temperature. Its inertness is crucial for the survival of many living organisms, as it prevents rapid oxidation reactions. Despite its relatively inert nature, nitrogen is essential for life, serving as a critical component of amino acids, proteins, and nucleic acids (DNA and RNA). The process of nitrogen fixation, where atmospheric nitrogen is converted into usable forms by microorganisms, is fundamental to the nitrogen cycle in ecosystems. Industrially, nitrogen is used in the production of ammonia, fertilizers, and various other chemical compounds.
Oxygen (O): The Essential Element for Life
Oxygen, the third most abundant element in the universe, is vital for the respiration of most living organisms. Its high reactivity is crucial for the processes that sustain life. It readily combines with other elements, forming oxides. Oxygen's role in combustion is essential for various industrial processes and energy generation. Medical applications of oxygen are crucial in emergency situations and for patients with respiratory problems. The importance of oxygen cannot be overstated in maintaining the life support systems on earth.
Chlorine (Cl): The Versatile Halogen
Chlorine, a halogen element, is a greenish-yellow gas at room temperature. It is highly reactive and a strong oxidizing agent. While known for its use in disinfectants and bleach, chlorine also plays a crucial role in the production of various chemicals, such as polyvinyl chloride (PVC), a common plastic. Its use in water purification is crucial for public health, effectively eliminating harmful bacteria and pathogens. While beneficial in these applications, chlorine must be handled carefully due to its toxic nature. High concentrations can be incredibly harmful to respiratory health.
Fluorine (F): The Most Reactive Element
Fluorine, the most reactive element in the periodic table, is a pale yellow gas at room temperature. Its reactivity necessitates specialized handling and storage procedures. Despite its reactivity, fluorine has several important applications. It is used in the production of various fluorocarbons, including Teflon (polytetrafluoroethylene), known for its non-stick properties and high chemical resistance. Fluorine is also a crucial component in the production of various pharmaceuticals and refrigerants. Its reactivity makes it a potent agent, but its use requires stringent safety measures.
Environmental Impact and Safety Considerations
The gaseous elements discussed above have a significant impact on the environment, both beneficial and detrimental. The noble gases, while generally inert, contribute to the overall atmospheric composition. However, their use in some applications can have indirect environmental consequences. For instance, helium, while non-toxic, is a non-renewable resource and its extraction and use require careful management to ensure sustainability.
Hydrogen, while a promising clean energy source, requires careful consideration of its production and storage, as some production methods may have substantial carbon footprints. Nitrogen, while essential for life, can contribute to environmental issues through its role in acid rain and eutrophication when released into the environment in excessive amounts. Oxygen, a vital element, is essential for life, but its overuse in industrial processes can contribute to depletion of resources.
Chlorine and fluorine, due to their high reactivity, must be handled with extreme care. Releases into the environment can have significant negative impacts on ecosystems and human health. Their presence in certain compounds, like chlorofluorocarbons (CFCs), has been linked to ozone depletion. Strict regulations and responsible use are essential for minimizing environmental damage and maintaining ecological balance.
Conclusion: Understanding the Gaseous Elements
The elements that exist as gases at room temperature represent a diverse group with a wide range of properties and applications. From the inert noble gases to the highly reactive halogens, these elements play crucial roles in various scientific, industrial, and environmental processes. Understanding their properties, uses, and environmental impact is essential for responsible scientific and technological advancement and for preserving the health of our planet. Continued research and innovation are necessary to optimize the use of these gases, minimizing negative environmental impacts and maximizing their benefits for society. As our understanding of these elements deepens, so will our ability to harness their potential while mitigating their risks.
Latest Posts
Latest Posts
-
The Size Of A Cell Is Limited By The
Apr 02, 2025
-
Explain The Role Of Organisms In The Carbon Cycle
Apr 02, 2025
-
Electric Field Due To Disk Of Charge
Apr 02, 2025
-
What Does Salt Do In Dna Extraction
Apr 02, 2025
-
Why Is Water Necessary For Life
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Elements That Are Gases At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
