Enthalpy Of Vaporization And Vapor Pressure Relationship
Muz Play
Mar 16, 2025 · 6 min read
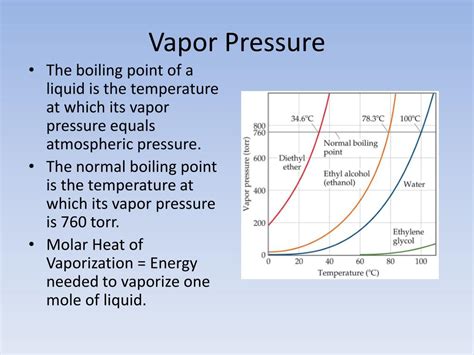
Table of Contents
Enthalpy of Vaporization and Vapor Pressure: A Deep Dive into the Relationship
The seemingly simple process of a liquid transforming into a gas, or vaporization, is governed by a complex interplay of thermodynamic properties. At the heart of this lies the relationship between enthalpy of vaporization and vapor pressure. Understanding this relationship is crucial in various fields, from chemical engineering and meteorology to pharmacy and material science. This article delves into the intricacies of this connection, exploring the underlying principles and their practical applications.
Understanding Enthalpy of Vaporization
Enthalpy of vaporization, often denoted as ΔH<sub>vap</sub>, represents the amount of heat energy required to convert one mole of a liquid substance into its gaseous phase at a constant temperature and pressure. This energy is primarily used to overcome the intermolecular forces holding the liquid molecules together. Stronger intermolecular forces, such as hydrogen bonds in water, result in higher enthalpy of vaporization values. Conversely, substances with weaker intermolecular forces, like noble gases, have lower ΔH<sub>vap</sub> values.
Factors Influencing Enthalpy of Vaporization
Several factors influence the enthalpy of vaporization of a substance:
- Intermolecular forces: As mentioned earlier, stronger intermolecular forces (hydrogen bonding, dipole-dipole interactions, London dispersion forces) necessitate more energy to overcome them, leading to a higher ΔH<sub>vap</sub>.
- Molecular weight: Heavier molecules generally have stronger London dispersion forces, resulting in higher enthalpy of vaporization.
- Molecular structure: The shape and size of molecules impact the extent of intermolecular interactions. Branched molecules, for instance, often have lower ΔH<sub>vap</sub> than their linear counterparts due to reduced surface area for interaction.
- Temperature: While ΔH<sub>vap</sub> is typically reported at the boiling point, it's important to note that its value varies slightly with temperature. Generally, ΔH<sub>vap</sub> decreases slightly as temperature increases.
Measuring Enthalpy of Vaporization
The enthalpy of vaporization can be experimentally determined using various techniques, including:
- Calorimetry: This involves measuring the heat absorbed during the vaporization process using a calorimeter. Precise temperature control and accurate heat capacity measurements are crucial for accurate results.
- Clausius-Clapeyron equation: This equation relates the enthalpy of vaporization to the vapor pressure at different temperatures (discussed in detail below). By measuring vapor pressure at multiple temperatures, ΔH<sub>vap</sub> can be calculated.
Delving into Vapor Pressure
Vapor pressure is the pressure exerted by a vapor in thermodynamic equilibrium with its condensed phases (solid or liquid) at a given temperature in a closed system. It's a measure of the tendency of a substance to transition from the liquid or solid phase to the gaseous phase. A higher vapor pressure indicates a greater tendency to evaporate.
Factors Affecting Vapor Pressure
Several factors influence the vapor pressure of a substance:
- Temperature: Vapor pressure increases exponentially with temperature. Higher temperatures provide molecules with greater kinetic energy, allowing more to escape the liquid phase.
- Intermolecular forces: Strong intermolecular forces restrict the escape of molecules from the liquid phase, resulting in lower vapor pressure.
- Molecular weight: Heavier molecules tend to have lower vapor pressures due to stronger intermolecular forces.
- Presence of other substances: The vapor pressure of a component in a solution is typically lower than its vapor pressure in the pure state (Raoult's Law).
Measuring Vapor Pressure
Vapor pressure can be measured using various methods, including:
- Manometry: This involves measuring the pressure difference between a closed container containing the liquid and a vacuum.
- Isoteniscope: This is a specialized apparatus designed for precise vapor pressure measurements.
- Gas chromatography: While primarily used for separating and analyzing mixtures, gas chromatography can indirectly provide vapor pressure information through retention time analysis.
The Intimate Relationship: Enthalpy of Vaporization and Vapor Pressure
The Clausius-Clapeyron equation beautifully encapsulates the relationship between enthalpy of vaporization and vapor pressure:
ln(P<sub>2</sub>/P<sub>1</sub>) = -ΔH<sub>vap</sub>/R * (1/T<sub>2</sub> - 1/T<sub>1</sub>)
where:
- P<sub>1</sub> and P<sub>2</sub> are the vapor pressures at temperatures T<sub>1</sub> and T<sub>2</sub> respectively.
- ΔH<sub>vap</sub> is the enthalpy of vaporization.
- R is the ideal gas constant.
This equation highlights the exponential relationship between vapor pressure and temperature, with the enthalpy of vaporization acting as a proportionality constant. A higher enthalpy of vaporization implies a steeper increase in vapor pressure with temperature. Conversely, a lower enthalpy of vaporization results in a gentler increase.
Practical Implications of the Relationship
The relationship between enthalpy of vaporization and vapor pressure has numerous practical applications:
- Predicting boiling points: The boiling point of a liquid is the temperature at which its vapor pressure equals the surrounding atmospheric pressure. Using the Clausius-Clapeyron equation and known ΔH<sub>vap</sub>, one can predict the boiling point at different pressures.
- Designing distillation processes: In chemical engineering, the efficient separation of liquid mixtures relies on understanding the vapor pressures of individual components, which are directly related to their enthalpy of vaporization.
- Understanding atmospheric phenomena: Meteorology utilizes this relationship to model weather patterns, as the vapor pressure of water in the atmosphere plays a crucial role in cloud formation and precipitation.
- Pharmaceutical applications: The vapor pressure of drugs influences their stability, delivery methods, and bioavailability. Understanding the enthalpy of vaporization aids in formulation development and optimization.
- Material science: The volatility of materials, a key property influenced by vapor pressure and enthalpy of vaporization, is critical in various applications, including coatings, adhesives, and polymer processing.
Advanced Considerations and Related Concepts
Beyond the fundamental relationship, several advanced concepts further elucidate the interplay between enthalpy of vaporization and vapor pressure:
- Critical point: At the critical point, the distinction between liquid and gas phases disappears. The enthalpy of vaporization approaches zero at this point, and vapor pressure reaches its maximum value.
- Trouton's rule: This rule provides an empirical estimate of the enthalpy of vaporization based on the boiling point. While a useful approximation, it has limitations, particularly for substances with strong hydrogen bonding or highly ordered liquid structures.
- Activity coefficients: In non-ideal solutions, the vapor pressure of a component deviates from Raoult's Law. Activity coefficients account for these deviations and are crucial in accurately predicting vapor pressures in complex mixtures.
Conclusion
The relationship between enthalpy of vaporization and vapor pressure is fundamental to understanding the behavior of liquids and their transition to the gaseous phase. This relationship, meticulously described by the Clausius-Clapeyron equation, has far-reaching implications across diverse scientific and engineering disciplines. From predicting boiling points to designing distillation processes and understanding atmospheric phenomena, a firm grasp of this connection is essential for solving real-world problems and advancing scientific knowledge. Further exploration into advanced concepts like the critical point and activity coefficients enriches our understanding and allows for even more precise predictions and applications. The continued study and application of these principles remain vital for progress in various scientific and technological fields.
Latest Posts
Latest Posts
-
What Is The Opposite Of Sublimation
Mar 17, 2025
-
Cellulose Is Composed Of Monomers Of
Mar 17, 2025
-
Find The Expansion Base Of N Formula
Mar 17, 2025
-
Can A Buffer Be Made With A Strong Acid
Mar 17, 2025
-
Gas Laws Practice Problems With Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Enthalpy Of Vaporization And Vapor Pressure Relationship . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
