Factors Affecting The Rate Of A Chemical Reaction Lab
Muz Play
Mar 23, 2025 · 6 min read
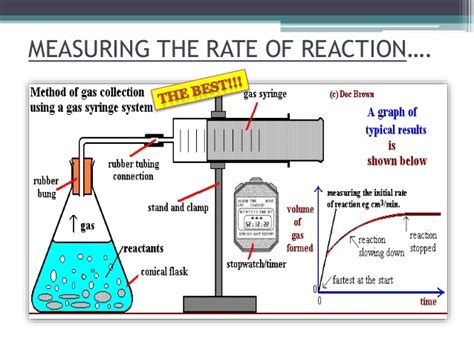
Table of Contents
Factors Affecting the Rate of a Chemical Reaction: A Comprehensive Lab Investigation
Understanding the factors that influence the rate of a chemical reaction is crucial in chemistry. This knowledge is vital not only for academic understanding but also for numerous industrial applications, from optimizing manufacturing processes to developing new catalysts. This article will delve deep into the key factors affecting reaction rates, providing a comprehensive overview suitable for both students and those with a general interest in chemistry. We will explore these factors through the lens of a hypothetical lab investigation, highlighting practical considerations and analysis techniques.
1. Concentration of Reactants
One of the most fundamental factors affecting reaction rate is the concentration of reactants. A higher concentration means more reactant particles are present in a given volume. This leads to a greater frequency of collisions between reactant particles, increasing the likelihood of successful collisions that lead to product formation.
1.1 The Collision Theory
The collision theory posits that for a reaction to occur, reactant particles must collide with sufficient energy (activation energy) and the correct orientation. Higher concentration directly increases the frequency of collisions, thus accelerating the reaction rate.
1.2 Lab Investigation: Concentration's Effect
In a lab setting, we could investigate this by reacting, for example, hydrochloric acid (HCl) with marble chips (calcium carbonate, CaCO₃). We would vary the concentration of HCl while keeping the mass of marble chips and temperature constant. By measuring the volume of carbon dioxide gas produced over time, we could observe the effect of concentration on the reaction rate. A higher concentration of HCl would lead to a faster initial rate of CO₂ production, as evidenced by a steeper initial slope on a graph of volume versus time.
2. Temperature
Temperature plays a critical role in reaction rates. Increasing the temperature increases the average kinetic energy of the reactant particles. This means more particles possess the necessary activation energy to overcome the energy barrier for reaction.
2.1 Activation Energy and the Arrhenius Equation
The activation energy (Ea) is the minimum energy required for a reaction to proceed. The Arrhenius equation, k = A * exp(-Ea/RT), mathematically relates the rate constant (k) to temperature (T), activation energy (Ea), and a pre-exponential factor (A). A higher temperature leads to a larger rate constant and thus a faster reaction rate.
2.2 Lab Investigation: Temperature's Influence
For our HCl and marble chips reaction, we could investigate the effect of temperature by performing the reaction at different temperatures (e.g., 20°C, 30°C, 40°C), again keeping the concentration and mass of reactants constant. We would observe a significant increase in the rate of CO₂ production with increasing temperature. Plotting the data allows for the determination of the activation energy using the Arrhenius equation.
3. Surface Area of Solids
When one or more reactants are solids, the surface area exposed to the other reactants significantly impacts the reaction rate. A larger surface area provides more sites for collisions to occur, increasing the reaction rate.
3.1 Particle Size and Reaction Rate
Finely divided solids have a much larger surface area compared to a single, large chunk of the same material. This explains why powdered reactants often react faster than their larger counterparts.
3.2 Lab Investigation: Surface Area's Role
To demonstrate this, we could react the same mass of marble chips with HCl, but use different sizes of marble chips—powdered, small chips, and large chunks. The powdered marble chips would react the fastest, followed by the small chips, and then the large chunks. This would be visually evident in the rate of CO₂ gas production.
4. Presence of a Catalyst
A catalyst is a substance that increases the rate of a chemical reaction without being consumed in the process. Catalysts achieve this by providing an alternative reaction pathway with a lower activation energy. This allows more reactant particles to possess the required energy for reaction, significantly increasing the rate.
4.1 Mechanism of Catalysis
Catalysts typically interact with reactants, forming intermediate compounds that subsequently decompose to yield the products and regenerate the catalyst. This lowers the overall activation energy for the reaction.
4.2 Lab Investigation: Catalytic Effect
The decomposition of hydrogen peroxide (H₂O₂) into water and oxygen is a classic example. This reaction is slow at room temperature, but the addition of manganese(IV) oxide (MnO₂) as a catalyst dramatically speeds up the decomposition, as evidenced by the vigorous bubbling of oxygen gas. A control experiment without the catalyst would show a much slower reaction rate.
5. Nature of Reactants
The nature of the reactants itself profoundly influences the reaction rate. Some reactions inherently proceed faster than others due to factors such as the strength of bonds involved and the complexity of the reaction mechanism.
5.1 Bond Strength and Reaction Rate
Reactions involving weaker bonds generally occur faster than those involving stronger bonds. This is because less energy is required to break weaker bonds.
5.2 Reaction Mechanism Complexity
The number of steps involved in a reaction mechanism also affects the overall rate. Reactions with complex multi-step mechanisms often proceed slower than those with simpler mechanisms.
5.3 Lab Investigation: Reactant Nature Comparison
Comparing the reaction rates of different reactants under similar conditions can highlight this factor. For instance, comparing the reaction rate of HCl with different metals (e.g., magnesium, zinc, iron) under controlled conditions would reveal different rates due to the varying reactivity of the metals.
6. Pressure (For Gaseous Reactions)
For reactions involving gaseous reactants, increasing the pressure increases the concentration of the gases. This effect is similar to the effect of increasing the concentration of liquid or solid reactants, leading to a higher frequency of collisions and a faster reaction rate.
6.1 Pressure and Collision Frequency
Higher pressure forces gas molecules closer together, increasing their collision frequency. This directly translates to a higher reaction rate.
6.2 Lab Investigation: Pressure's Influence
The reaction between nitrogen and hydrogen gases to form ammonia is a classic example. Increasing the pressure shifts the equilibrium towards the product side and increases the rate of ammonia formation.
7. Light (For Photochemical Reactions)
Certain reactions, known as photochemical reactions, require light to initiate the reaction. The intensity and wavelength of the light can significantly influence the reaction rate.
7.1 Light Absorption and Reaction Initiation
Light provides the energy necessary to overcome the activation energy barrier for photochemical reactions. Higher light intensity provides more energy, increasing the reaction rate. The wavelength of the light must also be appropriate for the reactants to absorb it effectively.
7.2 Lab Investigation: Light's Role
The photochemical decomposition of silver chloride (AgCl) is an example. Exposure to UV light causes AgCl to decompose into silver metal and chlorine gas. The rate of decomposition increases with increasing UV light intensity.
Conclusion
The rate of a chemical reaction is a complex interplay of various factors. By systematically investigating these factors through controlled experiments, as outlined in the proposed lab investigations, we can gain a deeper understanding of the principles governing chemical kinetics. This knowledge has broad implications across various scientific disciplines and technological advancements. Understanding these factors is not only essential for academic success but also for developing and optimizing chemical processes in various industries, including pharmaceuticals, manufacturing, and environmental science. Further research into each factor and the combined effects of multiple factors offers a rich field of exploration within chemistry.
Latest Posts
Latest Posts
-
In The Name Staphylococcus Aureus Aureus Is The
Mar 25, 2025
-
Absolute Value And Order Of Operations
Mar 25, 2025
-
How To Calculate The Heat Capacity Of The Calorimeter
Mar 25, 2025
-
What Is The Relationship Between Temperature And Pressure
Mar 25, 2025
-
Is Toxicity A Physical Or Chemical Property
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Factors Affecting The Rate Of A Chemical Reaction Lab . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
