How To Calculate The Heat Capacity Of The Calorimeter
Muz Play
Mar 25, 2025 · 6 min read
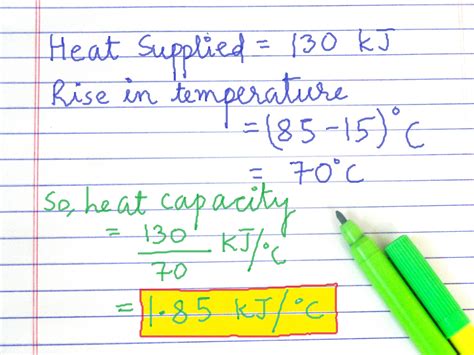
Table of Contents
How to Calculate the Heat Capacity of a Calorimeter: A Comprehensive Guide
Determining the heat capacity of a calorimeter is a crucial step in many calorimetry experiments. This value, often represented as C<sub>cal</sub>, represents the amount of heat required to raise the calorimeter's temperature by one degree Celsius (or one Kelvin). Accurately calculating C<sub>cal</sub> ensures precise measurements of heat transfer during reactions or phase transitions studied within the calorimeter. This comprehensive guide will walk you through the process, covering various methods, potential sources of error, and best practices for achieving reliable results.
Understanding Heat Capacity and Calorimetry
Before delving into the calculations, let's establish a clear understanding of the fundamental concepts:
What is Heat Capacity?
Heat capacity (C) is the ratio of the heat transferred (q) to the resulting temperature change (ΔT) of a substance or system. Mathematically, it's expressed as:
C = q / ΔT
The units are typically J/°C or J/K. The heat capacity of a substance depends on its mass, composition, and specific heat capacity.
What is a Calorimeter?
A calorimeter is an insulated device used to measure heat transfer during a process. Different types exist, including:
- Constant-pressure calorimeters (coffee-cup calorimeters): These operate at atmospheric pressure.
- Constant-volume calorimeters (bomb calorimeters): Used for reactions at constant volume, often combustion reactions.
Regardless of the type, the calorimeter itself absorbs some of the heat generated or absorbed during the process being studied. This heat absorption needs to be accounted for to accurately measure the heat associated solely with the reaction or process of interest.
Determining the Heat Capacity of the Calorimeter (C<sub>cal</sub>): The Method of Mixtures
The most common method for determining C<sub>cal</sub> involves a technique called the method of mixtures. This involves heating a known mass of water (or another substance with a known specific heat capacity) to a specific temperature and then transferring it to the calorimeter containing a known mass of water at a different temperature. The temperature change of the calorimeter and its contents are then measured.
Step-by-Step Procedure:
-
Measure the mass of water (m<sub>w</sub>) and its initial temperature (T<sub>w,initial</sub>) in the calorimeter. Use a precise balance and thermometer for accurate readings.
-
Heat a known mass of water (m<sub>h</sub>) to a significantly higher temperature (T<sub>h,initial</sub>). Ensure thorough and even heating to minimize temperature gradients within the heated water sample.
-
Carefully transfer the heated water into the calorimeter. Minimize heat loss to the surroundings during this transfer. This is often the most significant source of error.
-
Stir the mixture gently and continuously. This ensures thermal equilibrium between the calorimeter, the calorimeter's water, and the added water.
-
Monitor the temperature of the mixture (T<sub>final</sub>) until it stabilizes. Record this final temperature.
-
Calculate the heat lost by the hot water (q<sub>h</sub>):
q<sub>h</sub> = m<sub>h</sub> * c<sub>w</sub> * (T<sub>h,initial</sub> - T<sub>final</sub>)
where c<sub>w</sub> is the specific heat capacity of water (approximately 4.18 J/g°C).
-
Calculate the heat gained by the calorimeter and the calorimeter's water (q<sub>cal & w</sub>):
q<sub>cal & w</sub> = (m<sub>w</sub> * c<sub>w</sub> + C<sub>cal</sub>) * (T<sub>final</sub> - T<sub>w,initial</sub>)
This equation incorporates both the heat gained by the calorimeter's water and the heat gained by the calorimeter itself.
-
Equate the heat lost by the hot water to the heat gained by the calorimeter and its water:
q<sub>h</sub> = q<sub>cal & w</sub>
This assumes negligible heat loss to the surroundings.
-
Solve for C<sub>cal</sub>:
Substitute the expressions for q<sub>h</sub> and q<sub>cal & w</sub> into the equation above, and then solve for C<sub>cal</sub>. This involves some algebraic manipulation, but the result is a straightforward equation to calculate C<sub>cal</sub> based on the measured values.
Example Calculation:
Let's assume the following values were obtained in an experiment:
- m<sub>w</sub> = 50.0 g
- T<sub>w,initial</sub> = 25.0 °C
- m<sub>h</sub> = 50.0 g
- T<sub>h,initial</sub> = 70.0 °C
- T<sub>final</sub> = 42.5 °C
- c<sub>w</sub> = 4.18 J/g°C
First, calculate q<sub>h</sub>:
q<sub>h</sub> = (50.0 g) * (4.18 J/g°C) * (70.0 °C - 42.5 °C) = 2553.5 J
Next, calculate q<sub>cal & w</sub>:
q<sub>cal & w</sub> = [(50.0 g) * (4.18 J/g°C) + C<sub>cal</sub>] * (42.5 °C - 25.0 °C)
Now, equate q<sub>h</sub> and q<sub>cal & w</sub>:
2553.5 J = [(50.0 g) * (4.18 J/g°C) + C<sub>cal</sub>] * (17.5 °C)
Solve for C<sub>cal</sub>:
C<sub>cal</sub> = (2553.5 J / 17.5 °C) - (50.0 g * 4.18 J/g°C) ≈ 53.7 J/°C
Therefore, the heat capacity of the calorimeter in this example is approximately 53.7 J/°C.
Sources of Error and Minimization Strategies
Several factors can affect the accuracy of C<sub>cal</sub> determination. Minimizing these errors is crucial for reliable results:
-
Heat loss to the surroundings: This is often the most significant source of error. Proper insulation of the calorimeter is vital. The calorimeter should be well-insulated and the transfer of heated water must be performed quickly and efficiently.
-
Incomplete mixing: Ensure thorough mixing to achieve uniform temperature throughout the calorimeter. Inadequate mixing can lead to inaccurate temperature readings.
-
Inaccurate temperature measurements: Use calibrated thermometers or other precise temperature-measuring devices.
-
Heat from the thermometer: The thermometer itself may absorb or release some heat. Ensure that the thermometer is properly equilibrated before the measurements.
-
Evaporation of water: In open calorimeters, some water may evaporate, affecting the mass and heat transfer calculations. In such cases, consider using a lid to prevent or minimize evaporation.
Alternative Methods for Determining C<sub>cal</sub>
While the method of mixtures is the most common, other methods exist, often employed for specific calorimeter types:
-
Electrical heating method: A known amount of electrical energy is passed through a heating element within the calorimeter, raising the temperature. By measuring the change in temperature and electrical energy, C<sub>cal</sub> can be calculated using the principle of energy conservation.
-
Calibration with a standard reaction: A reaction with a known enthalpy change can be used. The enthalpy change, the mass of the reactants, and the temperature change in the calorimeter are used to determine C<sub>cal</sub>.
Conclusion
Calculating the heat capacity of a calorimeter is essential for accurate calorimetry experiments. The method of mixtures provides a relatively straightforward approach, but careful attention to detail and error minimization strategies are crucial for obtaining reliable results. Understanding the underlying principles of heat transfer and the limitations of different methods is vital for interpreting experimental data accurately and making valid conclusions. By adhering to the best practices and techniques outlined here, you can confidently determine C<sub>cal</sub> and perform precise calorimetric measurements.
Latest Posts
Latest Posts
-
Solving Linear Systems With Graphing 7 1
Mar 27, 2025
-
Compare And Contrast Sexual Reproduction And Asexual Reproduction
Mar 27, 2025
-
Interval Of Convergence For Taylor Series
Mar 27, 2025
-
The Martian And The Car Answer Key
Mar 27, 2025
-
Rusting Of Iron Chemical Or Physical Change
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How To Calculate The Heat Capacity Of The Calorimeter . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
