Five Postulates Of Dalton's Atomic Theory
Muz Play
Mar 18, 2025 · 6 min read
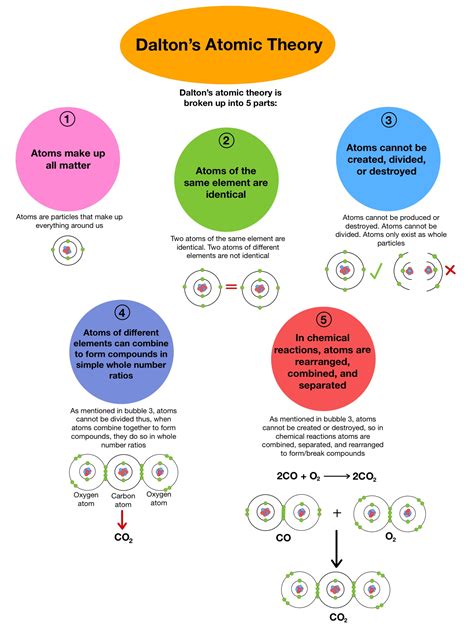
Table of Contents
Five Postulates of Dalton's Atomic Theory: A Deep Dive into the Foundation of Modern Chemistry
John Dalton's atomic theory, proposed in the early 1800s, revolutionized our understanding of matter. While some aspects have been refined or superseded by modern quantum mechanics, its core postulates remain foundational to chemistry. This article provides a comprehensive exploration of Dalton's five postulates, delving into their significance, limitations, and lasting impact on the field.
Postulate 1: All Matter is Made of Atoms
This seemingly simple statement was revolutionary at the time. Dalton posited that all matter, regardless of its form—solid, liquid, or gas—is composed of indivisible and indestructible particles called atoms. Before Dalton, the prevailing view was less precise, with philosophical discussions of matter often lacking a clear, quantitative framework. Dalton's assertion provided a concrete, foundational building block for understanding the physical world.
The Significance of Indivisibility
The concept of the atom as an indivisible particle was crucial. It implied that chemical reactions involved the rearrangement of atoms, not their creation or destruction. This provided a clear explanation for the law of conservation of mass—the observation that the total mass of reactants in a chemical reaction equals the total mass of the products. This fundamental principle wouldn't be fully explained until the discovery of nuclear reactions, which involve the conversion of mass to energy. Even then, Dalton's postulate remains a cornerstone for understanding everyday chemical transformations.
Beyond the Indivisible: Subatomic Particles
While Dalton believed atoms were indivisible, subsequent discoveries revealed a rich subatomic structure. Electrons, protons, and neutrons—particles far smaller than atoms—were discovered, demonstrating the complexity within what Dalton considered fundamental. This doesn't invalidate Dalton's postulate; rather, it highlights the iterative nature of scientific understanding. Dalton's work provided a crucial stepping stone, even if later findings provided a more nuanced picture. The idea of atoms as fundamental building blocks persists, even with the knowledge of their internal structure.
Postulate 2: All Atoms of a Given Element are Identical in Mass and Properties
Dalton proposed that all atoms of a particular element are exactly alike in mass and other properties. This postulate provided a way to differentiate between different elements. Each element was defined by its unique atomic mass. This seemingly straightforward statement laid the foundation for the periodic table, which organizes elements based on their atomic weight and recurring chemical properties.
Isotopes: A Refinement of the Postulate
Modern science understands that this postulate isn't entirely accurate. Isotopes, atoms of the same element with differing numbers of neutrons (and thus differing masses), exist. While isotopes of the same element have the same number of protons and electrons, leading to similar chemical properties, their differing neutron counts result in varying atomic masses. This discovery refined Dalton's understanding, demonstrating that atoms of the same element can have slightly different masses. Despite this refinement, the core concept—that elements are characterized by specific properties—remains essential to chemistry. The average atomic mass, considering the relative abundance of isotopes, is still a crucial concept.
Postulate 3: Atoms of Different Elements Differ in Mass and other Properties
This postulate directly builds upon the previous one. Dalton asserted that atoms of different elements possess distinct masses and properties. This is the cornerstone of distinguishing one element from another. The unique atomic mass of an element, along with its other unique characteristics (e.g., reactivity, melting point, etc.), distinguishes it from all other elements on the periodic table. This allows us to understand and predict chemical behavior based on the identity of the atoms involved.
The Importance of Distinctive Properties
The differences in atomic mass and properties are fundamental to chemical reactions. Atoms of different elements interact differently, forming compounds with unique characteristics based on the specific atoms involved. Understanding these differences is essential for predicting reaction outcomes and for designing new materials with desired properties. The periodic table itself is a testament to the success of this postulate in classifying and understanding the behavior of elements.
Postulate 4: Atoms Combine in Simple, Whole-Number Ratios to Form Compounds
This postulate explains the law of definite proportions (also known as the law of constant composition). This law states that a given chemical compound always contains the same elements in the same proportion by mass. For instance, water (H₂O) always contains two hydrogen atoms for every one oxygen atom. This principle is a direct consequence of atoms combining in whole-number ratios. You can't have half an atom of oxygen in a water molecule; it's either one or none.
The Implications for Chemical Formulas
This postulate is central to understanding chemical formulas. The subscripts in chemical formulas represent the whole-number ratios in which atoms combine. The formula for methane, CH₄, tells us that one carbon atom combines with four hydrogen atoms. This understanding is crucial for balancing chemical equations and predicting the amounts of reactants and products in chemical reactions. The simplicity of this postulate belies its profound implications for quantitative chemistry.
Postulate 5: Atoms Cannot be Created, Divided, or Destroyed in Chemical Reactions
This postulate relates to the law of conservation of mass. In chemical reactions, atoms are rearranged but not created or destroyed. This explains why the total mass of reactants always equals the total mass of products in a closed system. This is fundamentally different from nuclear reactions, where mass can be converted into energy (as described by Einstein's famous equation, E=mc²). However, for everyday chemical reactions, Dalton's postulate remains a remarkably accurate description.
The Persistence of Atoms Through Transformations
The rearrangement of atoms during chemical reactions explains the formation of new compounds. Chemical reactions involve the breaking and forming of chemical bonds, leading to changes in the arrangement of atoms, thus producing new substances with different properties. The atoms themselves, however, remain unchanged. This idea is essential for predicting the products of chemical reactions and understanding the stoichiometry (quantitative relationships) of chemical processes.
Limitations and Refinements of Dalton's Atomic Theory
While Dalton's atomic theory was groundbreaking, it has limitations:
- The indivisibility of atoms: As mentioned earlier, the discovery of subatomic particles demonstrated that atoms are not indivisible. They are composed of protons, neutrons, and electrons.
- Identical atoms of the same element: The existence of isotopes shows that atoms of the same element can have slightly different masses.
- Simple whole-number ratios: While true for many compounds, some compounds exhibit non-stoichiometric compositions, meaning their atomic ratios deviate slightly from simple whole numbers. This is often due to defects in the crystal structure of solids.
The Enduring Legacy of Dalton's Atomic Theory
Despite its limitations, Dalton's atomic theory remains a landmark achievement in the history of science. It provided a quantitative framework for understanding matter, paving the way for the development of modern chemistry. Its postulates, even with their refinements, continue to serve as the foundation of chemical principles. The concepts of atoms as fundamental building blocks, the unique properties of elements, and the conservation of mass during chemical reactions are all direct consequences of Dalton's revolutionary ideas.
The theory spurred further investigation, leading to advancements in understanding atomic structure, chemical bonding, and the behavior of matter at both the macroscopic and microscopic levels. Dalton's work, though incomplete, laid the groundwork for a deeper understanding of the universe, influencing generations of scientists and shaping the world we live in. His contributions are a testament to the power of observation, logical reasoning, and the persistent pursuit of scientific truth. The five postulates of Dalton's atomic theory, even with modern refinements, remain an essential part of the fundamental knowledge base of chemistry.
Latest Posts
Latest Posts
-
A Mixture That Forms When One Substance Dissolves Another
Mar 18, 2025
-
An Example Of An Extensive Property Of Matter Is
Mar 18, 2025
-
Spin Only Formula For Magnetic Moment
Mar 18, 2025
-
Spanish A1 Book Pdf Free Download
Mar 18, 2025
-
Is Coffee A Pure Substance Or Mixture
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Five Postulates Of Dalton's Atomic Theory . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
