Spin Only Formula For Magnetic Moment
Muz Play
Mar 18, 2025 · 6 min read
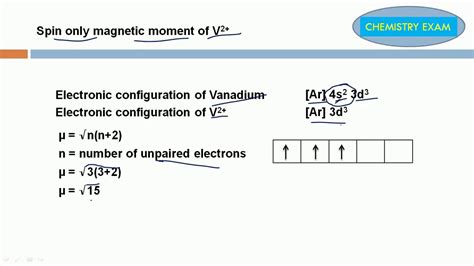
Table of Contents
Spin-Only Formula for Magnetic Moment: A Comprehensive Guide
Understanding the magnetic properties of materials is crucial in various fields, from materials science and chemistry to physics and engineering. One key aspect of this understanding involves calculating the magnetic moment of a substance, particularly for transition metal complexes. A simplified yet powerful tool for this calculation is the spin-only formula for magnetic moment. This article delves deep into the spin-only formula, explaining its derivation, applications, limitations, and the circumstances under which it provides accurate predictions.
What is Magnetic Moment?
Before diving into the spin-only formula, let's establish a fundamental understanding of magnetic moment. Magnetic moment is a measure of the strength and orientation of a magnetic field generated by a magnetic dipole. In the context of atoms and molecules, this magnetic field arises from the motion of electrons. These motions can be broadly categorized into two types: orbital motion and spin motion.
-
Orbital Magnetic Moment: Electrons orbiting the nucleus create a magnetic field analogous to a tiny current loop. This contributes to the overall magnetic moment of the atom or ion.
-
Spin Magnetic Moment: Electrons possess an intrinsic angular momentum called spin, which can be visualized as an electron spinning on its axis (though this is a simplified analogy). This spin is quantized, meaning it can only take on specific values. This spin generates a magnetic field and contributes to the overall magnetic moment.
The Spin-Only Formula: Derivation and Explanation
The spin-only formula provides a simplified estimate of the magnetic moment considering only the contribution from electron spin. It is particularly useful for transition metal complexes where the contribution from orbital angular momentum is often quenched or significantly reduced due to ligand field effects.
The formula is expressed as:
μ<sub>s</sub> = √[n(n+2)] BM
Where:
- μ<sub>s</sub> represents the spin-only magnetic moment, usually expressed in Bohr magnetons (BM).
- n represents the number of unpaired electrons.
- BM is the Bohr magneton, a physical constant representing the magnetic moment of an electron.
Derivation:
The derivation of this formula relies on quantum mechanics and involves the calculation of the total spin angular momentum (S) of the system. The total spin angular momentum is related to the number of unpaired electrons (n) as:
S = n/2
The spin-only magnetic moment is then calculated using the following equation derived from quantum mechanical principles:
μ<sub>s</sub> = g<sub>s</sub>√[S(S+1)] BM
Where:
- g<sub>s</sub> is the Lande g-factor for spin, approximately equal to 2.
Substituting S = n/2 into the above equation, we get:
μ<sub>s</sub> = 2√[(n/2)(n/2 + 1)] BM = √[n(n+2)] BM
This is the commonly used spin-only formula.
Applying the Spin-Only Formula: Step-by-Step Examples
Let's illustrate the application of the spin-only formula with a few examples.
Example 1: A Transition Metal Ion with Three Unpaired Electrons
Consider a transition metal ion with three unpaired electrons (n=3). Using the spin-only formula:
μ<sub>s</sub> = √[3(3+2)] BM = √15 BM ≈ 3.87 BM
The predicted spin-only magnetic moment is approximately 3.87 Bohr magnetons.
Example 2: A Complex with One Unpaired Electron
For a complex with only one unpaired electron (n=1):
μ<sub>s</sub> = √[1(1+2)] BM = √3 BM ≈ 1.73 BM
The predicted spin-only magnetic moment is approximately 1.73 Bohr magnetons.
Example 3: A Diamagnetic Complex
A diamagnetic complex possesses no unpaired electrons (n=0):
μ<sub>s</sub> = √[0(0+2)] BM = 0 BM
This confirms the absence of magnetic moment due to spin in diamagnetic species.
Limitations of the Spin-Only Formula
While the spin-only formula is a valuable tool, it's crucial to acknowledge its limitations:
-
Neglect of Orbital Contribution: The most significant limitation is its neglect of the orbital contribution to the magnetic moment. In some complexes, the orbital angular momentum is not completely quenched and contributes significantly to the overall magnetic moment. This is particularly true for complexes with low symmetry or those containing light transition metal ions.
-
Temperature Dependence: The formula doesn't explicitly account for temperature dependence. The magnetic moment can vary with temperature, especially in systems exhibiting spin-orbit coupling or significant zero-field splitting.
-
Accuracy Depends on the System: The accuracy of the spin-only formula heavily depends on the specific system under consideration. It works best for complexes with strong ligand fields that effectively quench orbital angular momentum.
When is the Spin-Only Formula Accurate?
The spin-only formula provides a reasonable approximation of the magnetic moment under certain conditions:
-
Strong Ligand Fields: Strong ligand fields lead to a significant reduction or complete quenching of orbital angular momentum. In these cases, the spin-only contribution dominates, and the formula is more accurate.
-
High-Spin Complexes: For high-spin complexes, the electron configuration often results in a larger number of unpaired electrons, leading to a stronger spin contribution and relatively less influence from orbital effects.
-
Octahedral Complexes of the Third-Row Transition Metals: Third-row transition metal ions often have relatively larger spin-orbit coupling effects, yet their large orbital contribution is usually significantly quenched in octahedral complexes making the spin only formula more valid compared to their lighter counterparts.
-
Comparison with Experimental Data: The ultimate test for the accuracy of the spin-only formula is comparison with experimental data obtained through techniques like magnetic susceptibility measurements. Discrepancies between the calculated and experimental values indicate the limitations of the spin-only approximation.
Beyond the Spin-Only Formula: Incorporating Orbital Contributions
When the spin-only formula proves inadequate, more sophisticated approaches are needed to account for orbital contributions. These methods often involve considering the effects of spin-orbit coupling and the detailed electronic structure of the complex using techniques like:
-
Effective Magnetic Moment Calculations: These involve correcting the spin-only formula to incorporate orbital contributions through various empirical or theoretical corrections.
-
Advanced Quantum Chemical Calculations: Sophisticated quantum chemical calculations can provide accurate predictions of the magnetic moment by explicitly considering all contributions from spin and orbital angular momentum. These calculations require extensive computational resources and expertise.
Conclusion
The spin-only formula serves as a valuable starting point for estimating the magnetic moment of transition metal complexes. It provides a simple yet useful tool for understanding the relationship between unpaired electrons and magnetic properties. However, its limitations must be carefully considered. Its accuracy significantly depends on the nature of the complex, the strength of the ligand field, and the presence of other effects. While it offers a reasonable approximation in certain cases, incorporating orbital contributions via more sophisticated methods may be necessary to achieve higher accuracy. The use of the spin-only formula should always be complemented by a critical evaluation of its applicability and a comparison with experimental magnetic data whenever possible. This holistic approach will yield a more comprehensive understanding of the magnetic behavior of coordination complexes.
Latest Posts
Latest Posts
-
What Is The Unit For Energy Flow
Mar 18, 2025
-
What Is Stationary Phase In Paper Chromatography
Mar 18, 2025
-
Is Ductile A Metal Or Nonmetal
Mar 18, 2025
-
Queremos Tus Palabras Oi Oyo Oyendo Oir
Mar 18, 2025
-
How To Find Profit Maximizing Price
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Spin Only Formula For Magnetic Moment . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
