What Is Stationary Phase In Paper Chromatography
Muz Play
Mar 18, 2025 · 6 min read
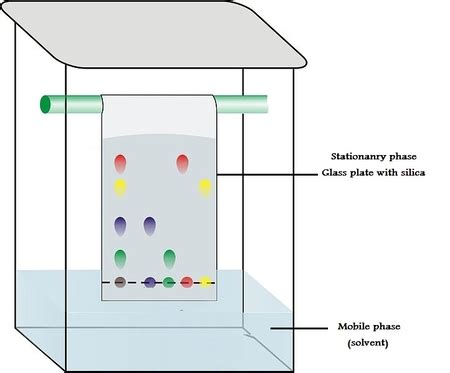
Table of Contents
What is the Stationary Phase in Paper Chromatography? A Deep Dive
Paper chromatography, a simple yet powerful analytical technique, is widely used to separate components of a mixture. Understanding its fundamental principles, especially the role of the stationary phase, is crucial for successful application. This comprehensive guide delves into the intricacies of the stationary phase in paper chromatography, exploring its properties, functions, and impact on separation efficiency.
Understanding the Basics of Paper Chromatography
Before diving into the stationary phase, let's establish a foundational understanding of paper chromatography. This technique leverages the differential partitioning of mixture components between two phases: a stationary phase and a mobile phase. The stationary phase remains fixed, while the mobile phase moves over it, carrying the mixture components. Different components interact differently with each phase, leading to their separation.
The separation process is governed by the partition coefficient (K), which represents the ratio of a component's concentration in the stationary phase to its concentration in the mobile phase. Components with higher K values exhibit stronger affinity for the stationary phase and move slower, while those with lower K values move faster.
The Stationary Phase: The Heart of the Separation
In paper chromatography, the stationary phase is the chromatographic paper itself. It's not simply inert paper; its properties significantly influence separation effectiveness. The paper's cellulose fibers possess numerous hydroxyl (-OH) groups, which are highly polar. These hydroxyl groups interact with the components of the mixture through various intermolecular forces like hydrogen bonding, dipole-dipole interactions, and van der Waals forces. This interaction dictates the retention time of each component on the paper.
Cellulose: The Key Player
The cellulose fibers forming the paper act as the primary component of the stationary phase. The unique structure of cellulose, with its long chains of glucose units and abundant hydroxyl groups, creates a complex network of pores and capillaries. This structure is critical for several reasons:
-
Capillary Action: The cellulose fibers' inherent capillary action draws the mobile phase upwards, creating the necessary flow for separation. This action is essential for the movement of the mixture components.
-
Adsorption and Partitioning: The hydroxyl groups on the cellulose fibers provide sites for adsorption and partitioning of the mixture components. Polar components interact strongly with these hydroxyl groups, leading to slower migration, while non-polar components interact less strongly and move faster.
-
Surface Area: The extensive surface area created by the fibrous structure of cellulose provides numerous interaction sites for the mixture components, improving separation efficiency.
Water's Role: More Than Just Moisture
The chromatographic paper is not completely dry; it contains a significant amount of adsorbed water within the cellulose fibers. This water layer plays a crucial role in the separation process, acting as an integral part of the stationary phase. The water molecules interact with the cellulose hydroxyl groups through hydrogen bonding, creating a hydrated cellulose layer.
The presence of this water layer further enhances the interaction between the stationary phase and the mixture components, especially polar ones. The separation is therefore not solely based on adsorption onto the cellulose fibers but also on partitioning between the mobile phase and the water layer within the cellulose matrix.
Modifying the Stationary Phase: Impregnation Techniques
The properties of the stationary phase can be modified to optimize separation for specific applications. One common technique involves impregnating the paper with various chemicals to alter its polarity and selectivity. For example:
-
Impregnation with Silica Gel: Incorporating silica gel into the paper creates a more polar stationary phase, suitable for separating non-polar components. The silica gel provides additional adsorption sites and alters the overall interaction characteristics.
-
Impregnation with Other Polar Solvents: Impregnation with other polar solvents, like formamide or glycerol, changes the stationary phase's polarity, impacting the separation of different classes of compounds.
Such modifications effectively tailor the stationary phase's properties to suit the specific requirements of the separation task, allowing for broader application of paper chromatography.
The Interaction between Stationary and Mobile Phases: A Dynamic Equilibrium
The separation process in paper chromatography involves a dynamic equilibrium between the stationary and mobile phases. As the mobile phase ascends the paper, the components of the mixture continuously partition between the two phases. A component with a high affinity for the stationary phase spends more time adsorbed on the cellulose fibers and moves slower. Conversely, a component with a lower affinity for the stationary phase spends less time adsorbed and migrates faster.
This continuous partitioning between the phases determines the final separation achieved. The effectiveness of the separation depends on the appropriate choice of both the stationary and mobile phases, ensuring optimal differences in their interaction with various components of the mixture.
Factors Affecting Separation Efficiency
Several factors influence the effectiveness of separation in paper chromatography, many directly related to the stationary phase:
-
Paper Quality: The quality of the chromatographic paper significantly affects separation. Uniform fiber distribution and consistency in the cellulose structure are crucial for reproducible results.
-
Moisture Content: The moisture content of the paper influences the stationary phase's polarity and affects the partitioning of components. Consistent moisture content is critical for reproducible results.
-
Temperature: Temperature changes affect the solubility and interactions of the components with the stationary phase. Controlled temperature is important for optimal separation.
-
Impregnation Technique: The method and extent of impregnation influence the stationary phase's properties, directly impacting the separation.
Optimizing these factors, along with the choice of the mobile phase, is essential for achieving high-quality separations in paper chromatography.
Applications of Paper Chromatography
The versatility of paper chromatography, largely due to the customizable properties of its stationary phase, makes it applicable to diverse fields:
-
Chemical Analysis: Separating and identifying components in mixtures, such as dyes, pigments, and amino acids.
-
Biochemical Analysis: Analyzing biological samples, like amino acids in proteins or sugars in carbohydrates.
-
Forensic Science: Identifying substances found at crime scenes.
-
Environmental Monitoring: Analyzing pollutants and contaminants in water or soil samples.
-
Education: A simple and accessible method for teaching fundamental principles of chromatography.
Conclusion: A Simple Technique with Profound Implications
The stationary phase in paper chromatography, primarily the cellulose fibers with their adsorbed water layer, is the foundation of this powerful separation technique. Its properties, including polarity, surface area, and moisture content, profoundly influence the separation efficiency. Understanding the intricacies of the stationary phase and how it interacts with the mobile phase is vital for successful application of paper chromatography across various scientific disciplines. By carefully controlling and manipulating the stationary phase properties, researchers can optimize the separation process to address specific analytical needs. The simplicity and affordability of paper chromatography, coupled with its remarkable versatility, solidify its continued relevance in both educational and professional settings.
Latest Posts
Latest Posts
-
Are Acids Proton Acceptors Or Donors
Mar 18, 2025
-
What Are Characteristics Of A Base
Mar 18, 2025
-
How To Calculate Expected Frequencies For Chi Square Test
Mar 18, 2025
-
What Is A Column In A Periodic Table
Mar 18, 2025
-
Final Product Of The Calvin Cycle
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is Stationary Phase In Paper Chromatography . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
