What Are Characteristics Of A Base
Muz Play
Mar 18, 2025 · 6 min read
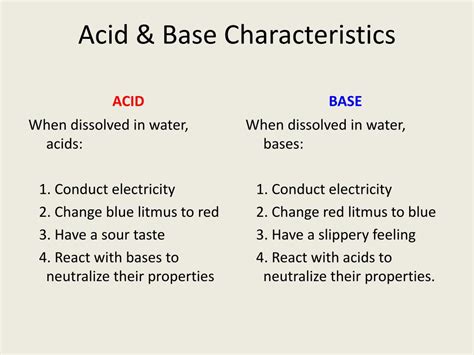
Table of Contents
What Are the Characteristics of a Base?
Understanding the characteristics of a base is fundamental to grasping numerous concepts in chemistry and related fields. Bases, alongside acids, form the cornerstone of acid-base chemistry, influencing reactions across various applications, from industrial processes to biological systems. This comprehensive guide dives deep into the defining characteristics of bases, exploring their properties, behaviors, and significance.
Defining Bases: More Than Just the Opposite of Acids
While often described as the "opposite" of acids, bases possess distinct and identifiable characteristics. Defining bases requires a multi-faceted approach, incorporating various theories and perspectives. Let's explore some key definitions:
Arrhenius Definition: The Hydroxide Ion Connection
The Arrhenius definition, one of the earliest approaches, defines a base as a substance that increases the hydroxide ion (OH⁻) concentration when dissolved in water. This definition is straightforward and applicable to many common bases, such as sodium hydroxide (NaOH) and potassium hydroxide (KOH). However, its limitation lies in its reliance on aqueous solutions; it doesn't encompass bases that don't involve hydroxide ions.
Brønsted-Lowry Definition: Proton Acceptors Take Center Stage
The Brønsted-Lowry definition offers a broader perspective. It defines a base as a proton (H⁺) acceptor. This definition expands the scope beyond aqueous solutions. A Brønsted-Lowry base doesn't necessarily need to produce hydroxide ions; it simply needs the ability to accept a proton from an acid. Ammonia (NH₃), for example, acts as a Brønsted-Lowry base by accepting a proton from water to form ammonium ions (NH₄⁺).
Lewis Definition: Electron Pair Donors
The Lewis definition presents the most comprehensive view of bases. It defines a base as an electron pair donor. This definition encompasses all Brønsted-Lowry bases and extends to substances that don't fit the previous definitions. A Lewis base provides a pair of electrons to form a coordinate covalent bond with an electron-deficient species (a Lewis acid). Many molecules and ions that don't contain hydroxide ions can act as Lewis bases, including ammonia, amines, and even some metal ions.
Key Characteristics of Bases: A Closer Look
Understanding the theoretical definitions is crucial, but recognizing the practical characteristics of bases is equally important. Several key properties help identify and differentiate bases:
1. Taste and Feel: Bitterness and Slippery Touch
Bases often exhibit a bitter taste and feel slippery or soapy to the touch. This characteristic is due to their reaction with the oils and proteins on our skin, forming soap-like substances. Caution: Never taste or touch unknown chemicals without proper safety precautions.
2. pH Greater Than 7: The pH Scale Indicator
One of the most reliable ways to identify a base is through its pH value. The pH scale ranges from 0 to 14, with 7 representing neutral. Bases have a pH greater than 7, with stronger bases exhibiting higher pH values closer to 14. The higher the pH, the more strongly basic the solution.
3. Reaction with Acids: Neutralization Reactions
Bases react with acids in a process called neutralization. This reaction typically produces salt and water. The reaction is exothermic, meaning it releases heat. The neutralization reaction is a fundamental concept in chemistry and is used in various applications, including titration, to determine the concentration of unknown solutions. For example, the reaction between sodium hydroxide (NaOH) and hydrochloric acid (HCl) yields sodium chloride (NaCl) and water (H₂O):
NaOH(aq) + HCl(aq) → NaCl(aq) + H₂O(l)
4. Electrical Conductivity: Ionic Conductors
Many bases, particularly strong bases, are good conductors of electricity when dissolved in water. This is because bases dissociate into ions in aqueous solutions, allowing the flow of electric current. The strength of conductivity depends on the degree of dissociation; strong bases dissociate completely, while weak bases dissociate partially.
5. Indicators: Revealing the Base's Presence
Various chemical indicators change color depending on the pH of a solution. These indicators are crucial in determining whether a substance is acidic, basic, or neutral. Litmus paper, for instance, turns blue in the presence of a base, providing a quick and simple test. Other indicators, such as phenolphthalein, undergo more dramatic color changes at specific pH ranges, offering more precise information.
6. Reaction with Metals: Specific Reactions with Amphoteric Metals
Certain metals, known as amphoteric metals, react with both acids and bases. Aluminum, zinc, and lead are examples of amphoteric metals. When these metals react with a base, they produce hydrogen gas and a salt. For instance, the reaction between aluminum and sodium hydroxide:
2Al(s) + 2NaOH(aq) + 6H₂O(l) → 2Na + 3H₂(g)
Classification of Bases: Strength and Weakness
Bases are classified based on their strength:
Strong Bases: Complete Dissociation
Strong bases completely dissociate into their constituent ions in aqueous solutions. This means that every molecule of the base releases its hydroxide ions (in the case of Arrhenius bases) into the solution. Examples include sodium hydroxide (NaOH), potassium hydroxide (KOH), calcium hydroxide (Ca(OH)₂), and barium hydroxide (Ba(OH)₂).
Weak Bases: Partial Dissociation
Weak bases only partially dissociate in water. This means that only a small fraction of the base molecules releases hydroxide ions (or accepts protons in the Brønsted-Lowry sense). Examples include ammonia (NH₃), pyridine (C₅H₅N), and many organic amines. The equilibrium between the undissociated base and its ions determines the strength of a weak base.
Applications of Bases: A Wide Range of Uses
Bases play a crucial role in numerous applications across various industries:
1. Industrial Applications: Manufacturing and Processing
Bases are essential in many industrial processes:
- Soap and Detergent Production: The saponification reaction, involving the reaction of fats and oils with strong bases, is fundamental to soap making.
- Paper Production: Bases are used in the pulping process to break down lignin, separating cellulose fibers.
- Textile Industry: Bases are used in dyeing and bleaching processes.
- Chemical Synthesis: Bases are employed as catalysts and reactants in various chemical synthesis reactions.
2. Biological Systems: Maintaining pH Balance
Bases are crucial in maintaining the pH balance in biological systems. Buffers, which are solutions containing a weak acid and its conjugate base (or a weak base and its conjugate acid), resist changes in pH. These buffers are vital for maintaining the proper pH in blood, cells, and other biological fluids. The human body utilizes bicarbonate buffers to regulate blood pH, ensuring optimal physiological function.
3. Everyday Life: From Cleaning to Cooking
Bases are commonly found in everyday products:
- Cleaning Agents: Many cleaning agents, such as ammonia and bleach, are alkaline solutions.
- Antacids: Antacids, used to relieve heartburn and indigestion, contain bases that neutralize stomach acid.
- Food Industry: Bases are used in baking and cooking to adjust the pH of ingredients and control the reaction rate.
Safety Precautions: Handling Bases with Care
Working with bases requires careful attention to safety measures:
- Protective Gear: Always wear appropriate protective gear, including gloves, goggles, and lab coats, when handling bases.
- Ventilation: Ensure adequate ventilation to avoid inhaling fumes.
- Neutralization: In case of spills, neutralize the base with a weak acid, such as vinegar, and then clean thoroughly.
- Disposal: Dispose of base solutions according to local regulations.
Conclusion: Understanding the Foundation of Basicity
The characteristics of bases, from their bitter taste and slippery feel to their crucial roles in various applications, highlight their significance in chemistry and beyond. Understanding the different definitions of bases, their classifications, and their safety precautions are essential for anyone working with or studying chemical systems. Their ability to accept protons, donate electron pairs, and affect pH levels makes them indispensable in countless industrial processes and biological systems. This comprehensive exploration provides a solid foundation for further investigation into this fascinating area of chemistry.
Latest Posts
Latest Posts
-
Converting Double Integrals To Polar Coordinates
Mar 19, 2025
-
How Do You Convert Moles To Volume
Mar 19, 2025
-
The Horizontal Columns On The Periodic Table Are Called
Mar 19, 2025
-
How To Find Derivative Of Limit
Mar 19, 2025
-
Proof Of The Parallel Axis Theorem
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Are Characteristics Of A Base . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
