Freezing Temp Of Water In Kelvin
Muz Play
Mar 16, 2025 · 5 min read
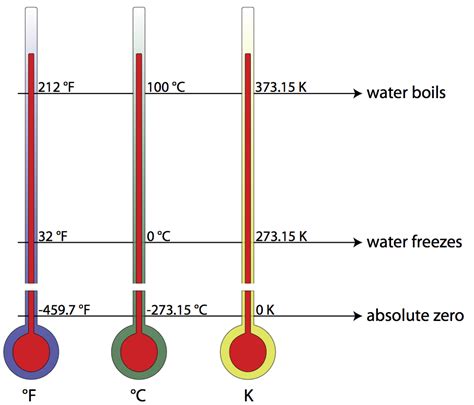
Table of Contents
The Freezing Point of Water in Kelvin: A Deep Dive
The freezing point of water, a seemingly simple concept, holds significant importance across various scientific disciplines and everyday life. Understanding its value, particularly in the Kelvin scale, unlocks a deeper appreciation for thermodynamics and the behavior of matter. This article delves into the intricacies of water's freezing point in Kelvin, exploring its scientific basis, practical applications, and the broader implications within the context of temperature scales.
Understanding Temperature Scales
Before diving into the specifics of water's freezing point in Kelvin, let's review the common temperature scales: Celsius (°C), Fahrenheit (°F), and Kelvin (K).
Celsius (°C)
The Celsius scale, widely used globally, sets the freezing point of water at 0°C and the boiling point at 100°C, under standard atmospheric pressure. Its simplicity and widespread adoption make it convenient for everyday applications.
Fahrenheit (°F)
Primarily used in the United States, the Fahrenheit scale defines water's freezing point at 32°F and boiling point at 212°F, again under standard atmospheric pressure. Its seemingly arbitrary values make it less intuitive for scientific work.
Kelvin (K)
The Kelvin scale, the absolute temperature scale, forms the cornerstone of thermodynamic calculations. It's based on absolute zero, the theoretical point where all molecular motion ceases. Zero Kelvin (0 K) is equivalent to -273.15°C or -459.67°F. The freezing point of water in Kelvin is 273.15 K. The scale uses the same degree increment as Celsius, meaning a change of 1 K is equal to a change of 1°C. This makes conversion between Kelvin and Celsius relatively straightforward.
The Significance of 273.15 K
The value 273.15 K, representing the freezing point of water, is not arbitrary. It's a fundamental constant derived from the properties of water and the definition of the Kelvin scale itself. This precise value allows for accurate calculations and predictions across numerous scientific fields.
Thermodynamics and Kinetic Energy
At 273.15 K, the kinetic energy of water molecules reaches a critical threshold. Below this temperature, the intermolecular forces of attraction (hydrogen bonds) dominate, causing the molecules to become rigidly ordered in a crystalline structure – ice. Above 273.15 K, the kinetic energy overcomes these forces, leading to the more disordered liquid state.
Phase Transitions and Enthalpy
The transition from liquid water to ice at 273.15 K involves a change in enthalpy, representing the heat absorbed or released during the phase change. This enthalpy change, known as the latent heat of fusion, is crucial in processes like ice formation and melting. Understanding this enthalpy change at 273.15 K is essential for various engineering applications.
Practical Applications of the Freezing Point of Water in Kelvin
The precise knowledge of water's freezing point in Kelvin (273.15 K) is vital in numerous fields:
Cryogenics and Low-Temperature Physics
Cryogenics involves the production and application of extremely low temperatures. Understanding the freezing point of water in Kelvin is crucial for designing and operating cryogenic systems, ensuring that water doesn't freeze unexpectedly and damage equipment. This is especially important in experiments involving superconductivity and other low-temperature phenomena.
Meteorology and Climatology
Meteorological and climatological models rely heavily on accurate temperature measurements. The freezing point of water in Kelvin provides a fundamental reference point for calibrating instruments and interpreting weather data. Accurate temperature readings are essential for predicting weather patterns, monitoring climate change, and understanding extreme weather events.
Food Science and Technology
In food science, the freezing point of water is critical for food preservation. Freezing food slows down or stops the growth of microorganisms, extending its shelf life. Understanding the freezing point in Kelvin helps in optimizing freezing processes to minimize ice crystal formation, which can damage food texture.
Chemistry and Materials Science
Many chemical reactions and material properties are temperature-dependent. Knowing the freezing point of water in Kelvin allows researchers to control reaction conditions precisely and study the behavior of materials at specific temperatures. This is vital for developing new materials with desired properties.
Environmental Science and Engineering
The freezing and thawing of water plays a crucial role in various environmental processes, such as erosion, hydrology, and ecosystem dynamics. Understanding the freezing point of water in Kelvin is essential for modeling these processes and assessing their impact on the environment. This is particularly relevant in areas affected by frost, permafrost, or glacial melt.
Factors Affecting the Freezing Point of Water
While 273.15 K is the standard freezing point of water under standard pressure, several factors can affect this value:
Pressure
Increasing pressure lowers the freezing point of water. This unusual property is due to the lower density of ice compared to liquid water. At high pressures, the liquid phase becomes more stable, and the freezing point shifts slightly below 273.15 K.
Impurities
Dissolved substances in water, such as salts or sugars, lower its freezing point. This phenomenon is known as freezing point depression and is the principle behind techniques like de-icing roads in winter. The extent of freezing point depression depends on the concentration of the dissolved impurities.
Isotopes
The isotopic composition of water can also influence its freezing point. Water molecules containing heavier isotopes of hydrogen (deuterium) or oxygen have slightly higher freezing points than "normal" water.
Conversion Between Temperature Scales
Converting between Celsius, Fahrenheit, and Kelvin requires understanding the relationships between these scales.
Celsius to Kelvin:
K = °C + 273.15
Kelvin to Celsius:
°C = K - 273.15
Celsius to Fahrenheit:
°F = (°C × 9/5) + 32
Fahrenheit to Celsius:
°C = (°F - 32) × 5/9
These conversion formulas are essential for accurately working with temperature values across different scales.
Conclusion
The freezing point of water in Kelvin, 273.15 K, is a fundamental constant with far-reaching implications across science, engineering, and everyday life. Its precise value allows for accurate calculations, predictions, and control in various applications, from cryogenics to food science. Understanding the factors that influence this value, coupled with the ability to convert between different temperature scales, is crucial for anyone working with temperature-dependent processes or phenomena. The seemingly simple concept of water freezing at 273.15 K unlocks a deeper understanding of the complex interactions within the physical world. The significance of this temperature transcends its simplicity, shaping our understanding of matter, energy, and the environment.
Latest Posts
Latest Posts
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
-
A Temporary Mixture The Particles Will Eventually Settle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Freezing Temp Of Water In Kelvin . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
