Gas In A Gas Solution Example
Muz Play
Mar 31, 2025 · 6 min read
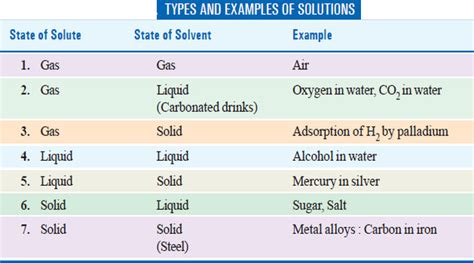
Table of Contents
Gas in a Gas Solution: A Deep Dive into Composition, Behavior, and Applications
Understanding gas solubility in gas solutions might seem counterintuitive at first. After all, gases are already in a gaseous state. However, the concept refers to the dissolution of one gas into another, a phenomenon governed by principles of partial pressure, temperature, and intermolecular forces. This exploration delves into the intricacies of gas-in-gas solutions, examining real-world examples, influencing factors, and practical applications across various industries.
What is a Gas in a Gas Solution?
A gas in a gas solution, also known as a gaseous solution, is a homogeneous mixture where one gas dissolves into another. Unlike solutions involving liquids or solids, the solvent and solute are both in the gaseous phase. The key distinction lies in the differing concentrations of the components. The gas present in a higher concentration acts as the solvent, while the gas present in a lower concentration acts as the solute. The gases mix spontaneously due to the kinetic energy of their constituent molecules and the attractive forces (though weak) between them.
Key Characteristics of Gas-in-Gas Solutions:
- Homogeneity: The gases are uniformly dispersed throughout the mixture, forming a single phase.
- Variable Composition: The ratio of gases can vary widely, depending on the partial pressures and conditions.
- No Chemical Reactions: The gases generally do not react chemically with each other; they simply mix physically.
- Dependence on Partial Pressure: The solubility of a gas in another gas is directly proportional to its partial pressure (Dalton's Law of Partial Pressures).
Examples of Gas in a Gas Solutions:
The air we breathe provides the most prevalent example of a gas in a gas solution.
1. Air: A Classic Example
Air is a complex mixture primarily composed of nitrogen (approximately 78%), oxygen (approximately 21%), and trace amounts of argon, carbon dioxide, and other gases. Nitrogen acts as the dominant solvent, with oxygen and the other gases dissolved within it. The concentrations of these gases remain relatively constant in the troposphere due to atmospheric mixing, but can vary at higher altitudes.
2. Natural Gas: A Mixture of Hydrocarbons
Natural gas is another significant example. It's primarily composed of methane (CH₄), but also contains varying amounts of ethane, propane, butane, and other hydrocarbon gases. These gases are mutually soluble, creating a homogeneous mixture that's used extensively as a fuel source. The composition of natural gas varies depending on its source.
3. Industrial Gas Mixtures: Tailored for Specific Applications
Many industrial processes require specific gas mixtures tailored to their needs. For instance, protective atmospheres in welding or heat treatment frequently involve mixtures of nitrogen, argon, and carbon dioxide. The precise composition is adjusted to control the oxidation and decarburization of the metal being processed. Similarly, the semiconductor industry utilizes meticulously controlled gas mixtures during various fabrication stages.
4. Breathing Mixtures for Divers: Optimizing Gas Solubility for Depth
Divers' breathing mixtures, particularly at significant depths, often include gases other than air to minimize the risk of decompression sickness. These mixtures, often involving helium, oxygen, and nitrogen in varying proportions, are designed to manage the solubility of gases in the diver's blood at different pressures. Helium, for example, has a lower solubility than nitrogen, reducing the risk of gas bubbles forming during ascent.
Factors Affecting Gas Solubility in Gas Solutions:
Several factors govern the extent to which one gas dissolves in another. Understanding these factors is crucial for controlling and predicting the behavior of gaseous solutions.
1. Partial Pressure: The Dominant Factor
Partial pressure plays the most significant role in determining gas solubility. According to Dalton's Law of Partial Pressures, the total pressure of a gas mixture is the sum of the partial pressures of its individual components. The solubility of a gas is directly proportional to its partial pressure; higher partial pressures lead to greater solubility. This is why increasing the pressure on a gas mixture increases the amount of each gas dissolved within the solution.
2. Temperature: A Complex Relationship
The effect of temperature on gas solubility in gas solutions is less straightforward than in liquid solutions. Generally, increasing the temperature increases the kinetic energy of the gas molecules, making them less likely to interact and dissolve into each other. However, this relationship is not always linear and can be significantly influenced by the specific gases involved and the intermolecular forces between them.
3. Intermolecular Forces: Weak but Significant
While weak compared to those in liquid or solid solutions, intermolecular forces between gas molecules still impact solubility. Similar molecular structures and polarities can lead to stronger attractive forces between gases, slightly enhancing solubility. However, these forces are usually much weaker than the kinetic energy of the molecules, making their influence secondary to partial pressure and temperature.
4. Molecular Size and Shape: A Subtle Influence
The size and shape of the gas molecules can also subtly affect their solubility. Larger, more complex molecules may interact less effectively with other gases due to steric hindrance, potentially reducing solubility.
Applications of Gas in Gas Solutions:
The concept of gas in gas solutions finds applications in diverse fields.
1. Respiratory Care: Tailoring Breathing Mixtures
In respiratory care, understanding gas solubility is vital for designing breathing mixtures for patients with respiratory problems. The concentrations of oxygen and other gases are adjusted based on the patient's individual needs and the conditions being treated.
2. Chemical Processes: Controlled Atmospheres
Numerous chemical processes rely on carefully controlled gaseous atmospheres. For instance, the synthesis of certain chemicals requires a specific mixture of gases to maintain the desired reaction conditions and prevent unwanted side reactions.
3. Food Packaging: Modified Atmosphere Packaging (MAP)
Modified Atmosphere Packaging (MAP) is extensively used in the food industry to extend the shelf life of products. Replacing the air inside the packaging with a gas mixture, often involving nitrogen, carbon dioxide, and sometimes oxygen, slows down spoilage by inhibiting microbial growth and oxidation.
4. Welding and Metalworking: Protective Atmospheres
Welding and other metalworking processes often use controlled gas mixtures to protect the workpiece from oxidation and other undesirable reactions. These atmospheres are carefully selected based on the metal being processed and the desired properties of the final product.
5. Environmental Science: Atmospheric Modelling and Air Quality Monitoring
Understanding gas solubility is crucial for atmospheric modelling and air quality monitoring. Accurate models require an understanding of how various gases interact and dissolve within the atmosphere, which impacts pollutant dispersion and climate change projections.
Conclusion: The Subtle Complexity of Gaseous Solutions
Gas in a gas solution, while seemingly simple, presents a fascinating interplay of physical and chemical principles. The dominance of partial pressure, the nuanced role of temperature and intermolecular forces, and the varied applications across numerous industries underscore the importance of understanding this fundamental aspect of chemistry. Further research into the intricacies of gas solubility continues to refine our ability to predict and control the behavior of gaseous mixtures, leading to advancements in various fields, from respiratory care to environmental science. The seemingly simple act of breathing air itself stands as a testament to the remarkable complexity and significance of gas-in-gas solutions.
Latest Posts
Latest Posts
-
How Does The Nucleus And Ribosomes Work Together
Apr 01, 2025
-
Having A Single Set Of Unpaired Chromosomes
Apr 01, 2025
-
How To Calculate Current In A Series Circuit
Apr 01, 2025
-
Unity Of Life And Diversity Of Life
Apr 01, 2025
-
Choose The Most Likely Correlation Value For This Scatterplot
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Gas In A Gas Solution Example . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
