Gay Lussac Law Of Combining Volume
Muz Play
Mar 18, 2025 · 5 min read
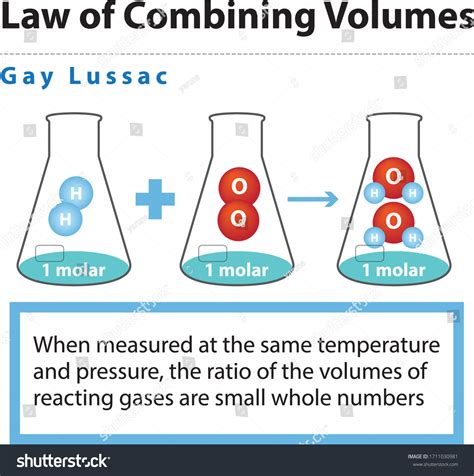
Table of Contents
Gay-Lussac's Law of Combining Volumes: A Deep Dive into Gas Reactions
Gay-Lussac's Law of Combining Volumes, a cornerstone of chemistry, elegantly describes the relationships between the volumes of gases involved in chemical reactions. Unlike other gas laws focusing on pressure, temperature, or volume changes in a single gas, Gay-Lussac's law specifically examines the ratios of gas volumes reacting and the volumes of gaseous products formed. This article will explore the law itself, its historical context, its limitations, and its vital role in establishing the atomic theory.
Understanding Gay-Lussac's Law
Simply stated, Gay-Lussac's Law of Combining Volumes states that when gases react, they do so in volumes which bear a simple ratio to one another and to the volumes of the gaseous products, provided that all measurements are made at the same temperature and pressure. This "simple ratio" is often expressed as small whole numbers like 1:1, 1:2, 2:3, and so on. It's crucial to remember the condition of constant temperature and pressure; deviations will occur otherwise.
Consider the reaction between hydrogen and oxygen to produce water vapor:
2H₂(g) + O₂(g) → 2H₂O(g)
According to Gay-Lussac's law, if we react 2 volumes of hydrogen gas with 1 volume of oxygen gas (at the same T and P), we will obtain 2 volumes of water vapor. The ratio is a simple 2:1:2. This observation holds true for numerous other gas reactions. The simplicity of the volume ratios strongly suggests a fundamental relationship between the constituent particles of gases.
Examples Illustrating Gay-Lussac's Law
Several examples effectively highlight the application of Gay-Lussac's Law:
- Reaction of Hydrogen and Chlorine: Hydrogen gas (H₂) reacts with chlorine gas (Cl₂) to form hydrogen chloride gas (HCl). The reaction can be represented as:
H₂(g) + Cl₂(g) → 2HCl(g)
One volume of hydrogen reacts with one volume of chlorine to produce two volumes of hydrogen chloride. The volume ratio is 1:1:2.
- Reaction of Nitrogen and Hydrogen: Nitrogen gas (N₂) reacts with hydrogen gas (H₂) to produce ammonia gas (NH₃):
N₂(g) + 3H₂(g) → 2NH₃(g)
One volume of nitrogen reacts with three volumes of hydrogen to produce two volumes of ammonia. The volume ratio here is 1:3:2.
These examples, along with numerous others, consistently demonstrate the simple whole-number ratios predicted by Gay-Lussac's law. This regularity strongly implied an underlying order in the structure of matter.
Historical Context and Avogadro's Hypothesis
Gay-Lussac's law, published in 1808, significantly influenced the development of atomic theory. While the law itself described the observed phenomenon, it lacked a complete theoretical explanation. That explanation came from Amedeo Avogadro, who proposed his hypothesis in 1811.
Avogadro's Hypothesis states that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This hypothesis provided the crucial link needed to understand Gay-Lussac's law. By combining Gay-Lussac's experimental observations with Avogadro's hypothesis, scientists could finally deduce the relative number of atoms in molecules participating in reactions.
For instance, in the hydrogen-oxygen reaction, Avogadro's hypothesis implies that 2 volumes of hydrogen contain twice the number of molecules as 1 volume of oxygen. This suggested that the hydrogen molecule (H₂) consists of two hydrogen atoms, and the oxygen molecule (O₂) consists of two oxygen atoms. This was a revolutionary insight into the structure of molecules.
Limitations of Gay-Lussac's Law
While powerful and insightful, Gay-Lussac's law does possess limitations:
-
Applies only to gases: The law is applicable only to reactions involving gases. It does not apply to reactions involving liquids or solids.
-
Ideal gas behavior: The law assumes ideal gas behavior. At high pressures or low temperatures, real gases deviate from ideal behavior, and the simple volume ratios may not hold perfectly.
-
Reactions with solids or liquids: If reactants or products are not gases, the law is not applicable in its simple form. For instance, consider the combustion of methane:
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
While the gaseous reactants and products still show simple volume relationships, the inclusion of water (initially a gas at high temperature, but possibly liquid at lower temperature) complicates things.
- Unreactive gases: Inert or noble gases like helium or neon which don't actively participate in chemical reactions, are an obvious example of a limitation. The law doesn't apply to these gases in isolation.
Despite these limitations, Gay-Lussac's law provides a valuable framework for understanding gaseous reactions and remains a crucial element in the foundation of chemical principles.
Gay-Lussac's Law and Modern Chemistry
Today, Gay-Lussac's law is not used directly for quantitative calculations of reaction yields. More sophisticated methods based on the ideal gas law (PV=nRT) and stoichiometry provide a more accurate and comprehensive approach. However, its significance remains profound.
The law's historical impact on the development of atomic theory cannot be overstated. It provided essential experimental evidence supporting the existence of atoms and molecules and helped scientists determine the relative number of atoms in various molecules. This foundational understanding paved the way for advancements in countless areas of chemistry, including:
-
Understanding molecular structure: The law contributed significantly to our ability to determine the structure and composition of molecules.
-
Development of stoichiometry: The principle of combining volumes directly influenced the development of stoichiometry, enabling the quantitative analysis of chemical reactions.
-
Development of the Ideal Gas Law: The knowledge gained from studying the volume relationships in gas reactions helped pave the way for a more comprehensive understanding of gas behavior, ultimately culminating in the Ideal Gas Law.
-
Industrial applications: Understanding volume relationships in gas reactions remains crucial in many industrial chemical processes, ensuring efficient and optimized reaction conditions.
Conclusion: A Legacy of Discovery
Gay-Lussac's Law of Combining Volumes, though possessing some limitations, remains a landmark achievement in the history of chemistry. Its contribution to our understanding of molecular structure, stoichiometry, and the behavior of gases is undeniable. While modern chemical calculations utilize more advanced techniques, Gay-Lussac's law serves as a powerful testament to the importance of experimental observation and its profound influence on theoretical development in the field of chemistry. The simple elegance of its statement and the revolutionary consequences of its discovery continue to inspire and educate scientists and students alike. It represents a pivotal step in the long journey of human understanding of the material world.
Latest Posts
Latest Posts
-
An Intermediate Phenotype Indicates That A Trait Has Dominance
Mar 19, 2025
-
Plant Is Where Photosynthesis Takes Place
Mar 19, 2025
-
The Force Driving Plate Tectonics Is
Mar 19, 2025
-
Converting Double Integrals To Polar Coordinates
Mar 19, 2025
-
How Do You Convert Moles To Volume
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Gay Lussac Law Of Combining Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
