Gibbs Free Energy From Cell Potential
Muz Play
Mar 16, 2025 · 6 min read
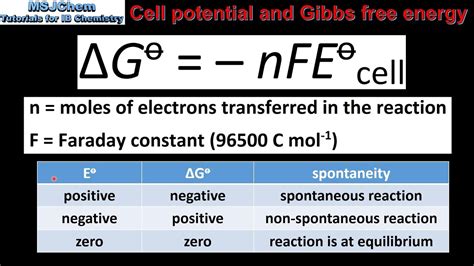
Table of Contents
Gibbs Free Energy from Cell Potential: A Deep Dive into Thermodynamics and Electrochemistry
The world of electrochemistry is fascinating, bridging the gap between chemical reactions and electrical energy. At the heart of this connection lies the Gibbs Free Energy (ΔG), a thermodynamic quantity that predicts the spontaneity of a reaction, and the cell potential (E°cell), a measure of the electromotive force driving the reaction in an electrochemical cell. Understanding the relationship between these two crucial parameters is key to comprehending the behavior of electrochemical systems. This article delves deep into the connection between Gibbs Free Energy and cell potential, exploring its implications and applications.
Understanding Gibbs Free Energy
Gibbs Free Energy, denoted as ΔG, represents the maximum amount of reversible work that can be done by a system at constant temperature and pressure. A negative ΔG indicates a spontaneous reaction, meaning the reaction will proceed without external intervention. A positive ΔG signifies a non-spontaneous reaction, requiring energy input to proceed. A ΔG of zero indicates a system at equilibrium, where the forward and reverse reaction rates are equal. The Gibbs Free Energy is calculated using the equation:
ΔG = ΔH - TΔS
where:
- ΔG is the change in Gibbs Free Energy
- ΔH is the change in enthalpy (heat content)
- T is the temperature in Kelvin
- ΔS is the change in entropy (disorder)
Understanding the individual components – enthalpy and entropy – is crucial for interpreting the overall spontaneity of a reaction. Enthalpy reflects the heat exchange during a reaction; exothermic reactions (ΔH < 0) release heat, while endothermic reactions (ΔH > 0) absorb heat. Entropy measures the randomness or disorder of a system; an increase in entropy (ΔS > 0) indicates a more disordered state, while a decrease (ΔS < 0) indicates a more ordered state.
Cell Potential and Electromotive Force
An electrochemical cell, also known as a galvanic cell or voltaic cell, is a device that generates electricity from a spontaneous redox reaction. The cell potential, E°cell, is a measure of the driving force of this reaction. It represents the difference in electrical potential between the two electrodes (anode and cathode) in the cell under standard conditions (298 K, 1 atm pressure, 1 M concentration). A positive cell potential indicates a spontaneous reaction (positive electromotive force), while a negative cell potential signifies a non-spontaneous reaction (negative electromotive force) requiring an external power source to drive it.
The cell potential is calculated using the Nernst equation:
E<sub>cell</sub> = E°<sub>cell</sub> - (RT/nF)lnQ
where:
- E<sub>cell</sub> is the cell potential under non-standard conditions
- E°<sub>cell</sub> is the standard cell potential
- R is the ideal gas constant (8.314 J/mol·K)
- T is the temperature in Kelvin
- n is the number of moles of electrons transferred in the balanced redox reaction
- F is the Faraday constant (96485 C/mol)
- Q is the reaction quotient
The Nernst equation accounts for the influence of non-standard conditions (concentrations, pressure, temperature) on the cell potential. At standard conditions, Q = 1, and the equation simplifies to E<sub>cell</sub> = E°<sub>cell</sub>.
Connecting Gibbs Free Energy and Cell Potential
The fundamental relationship between Gibbs Free Energy and cell potential is given by the equation:
ΔG = -nFE°<sub>cell</sub>
This powerful equation directly links thermodynamics (ΔG) and electrochemistry (E°<sub>cell</sub>). A negative ΔG corresponds to a positive E°<sub>cell</sub>, indicating a spontaneous reaction. Conversely, a positive ΔG corresponds to a negative E°<sub>cell</sub>, signifying a non-spontaneous reaction. The magnitude of ΔG is proportional to the magnitude of E°<sub>cell</sub>; a larger cell potential implies a greater driving force for the reaction and a more negative Gibbs Free Energy.
This equation provides a crucial tool for predicting the spontaneity and determining the equilibrium constant of a redox reaction. By measuring the cell potential, we can directly calculate the Gibbs Free Energy change, gaining valuable insights into the reaction's thermodynamics.
Applications and Implications
The relationship between Gibbs Free Energy and cell potential has numerous applications across various fields:
1. Predicting Reaction Spontaneity:
By calculating the cell potential, we can easily predict whether a redox reaction will occur spontaneously under given conditions. This is crucial in designing and optimizing electrochemical devices, such as batteries and fuel cells.
2. Determining Equilibrium Constants:
The Gibbs Free Energy is related to the equilibrium constant (K) by the equation:
ΔG° = -RTlnK
Combining this with the equation relating ΔG and E°<sub>cell</sub> allows us to calculate the equilibrium constant from the standard cell potential. This provides valuable information about the extent to which a redox reaction will proceed to completion.
3. Battery Design and Performance:
The cell potential is a direct measure of the voltage a battery can provide. By understanding the relationship between Gibbs Free Energy and cell potential, battery designers can optimize the materials and design to achieve the desired voltage and energy capacity. Higher cell potentials translate to higher energy densities in batteries.
4. Corrosion Studies:
Corrosion is an electrochemical process involving the oxidation of metals. By analyzing the cell potential associated with corrosion reactions, we can predict the likelihood and rate of corrosion under specific conditions. This knowledge is essential in developing corrosion-resistant materials and protective coatings.
5. Fuel Cell Technology:
Fuel cells generate electricity by electrochemically oxidizing a fuel, typically hydrogen. The cell potential determines the efficiency of the fuel cell, with higher cell potentials indicating greater energy conversion efficiency. Understanding the thermodynamic aspects helps in improving fuel cell design and performance.
6. Electroplating and Electrolysis:
Electroplating and electrolysis are non-spontaneous electrochemical processes that require an external power source. By knowing the Gibbs Free Energy and cell potential, the required voltage and current can be precisely determined to control the process effectively.
Beyond Standard Conditions: The Nernst Equation and its Significance
The relationship ΔG = -nFE°<sub>cell</sub> holds true only under standard conditions. For non-standard conditions, we must utilize the Nernst equation to calculate the cell potential and subsequently determine the Gibbs Free Energy. The Nernst equation incorporates the effects of concentration, pressure, and temperature on the cell potential. Therefore, understanding the Nernst equation is crucial for accurate predictions of spontaneity and equilibrium under real-world conditions.
The Nernst equation reveals that the cell potential deviates from the standard cell potential as the reaction quotient (Q) deviates from unity. As Q increases (products become more favored), the cell potential decreases, indicating a less favorable driving force. Conversely, as Q decreases (reactants become more favored), the cell potential increases, indicating a more favorable driving force.
Conclusion: A Powerful Interplay
The connection between Gibbs Free Energy and cell potential offers a powerful tool for understanding and manipulating electrochemical systems. This relationship allows us to predict the spontaneity of redox reactions, determine equilibrium constants, and design and optimize electrochemical devices. By combining thermodynamic principles with electrochemical measurements, we gain a deeper understanding of the energy transformations occurring within these systems. The ability to predict and control these transformations has broad applications across various scientific and technological fields, from energy storage and conversion to materials science and environmental protection. Further exploration of this relationship continues to pave the way for innovative advances in these areas.
Latest Posts
Latest Posts
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
-
Evidence Of Light As A Particle
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Gibbs Free Energy From Cell Potential . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
