Ground State Electron Configuration For Na
Muz Play
Mar 16, 2025 · 6 min read
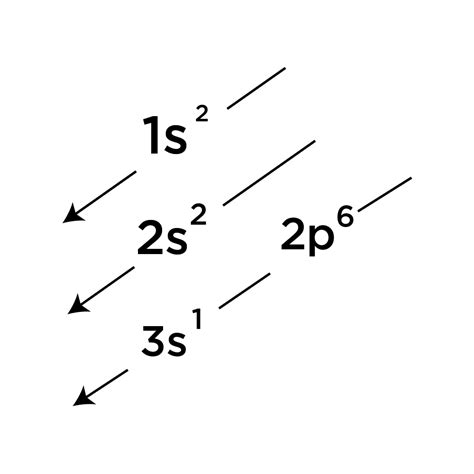
Table of Contents
Ground State Electron Configuration for Na: A Deep Dive
Sodium (Na), a ubiquitous alkali metal, provides a fantastic entry point for understanding electron configurations and their implications in chemistry. This article will delve deep into the ground state electron configuration of sodium, explaining the principles behind it, its significance, and its connection to the element's properties. We'll explore the concepts using various notations, address exceptions, and highlight the importance of understanding electron configurations in predicting chemical behavior.
Understanding Electron Configurations
Before diving into sodium's specific configuration, let's establish a foundational understanding of electron configuration itself. An electron configuration describes the arrangement of electrons in an atom's energy levels and sublevels. It dictates how electrons are distributed amongst various orbitals, influencing an atom's reactivity and chemical bonding capabilities. These arrangements adhere to specific rules governed by quantum mechanics.
The Aufbau Principle
The Aufbau principle, which translates to "building-up principle," guides the filling of orbitals. Electrons populate orbitals starting with the lowest energy levels and progressively moving to higher energy levels. This principle is fundamental in predicting ground state electron configurations.
Hund's Rule
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any single orbital. This minimizes electron-electron repulsion, leading to a more stable configuration. Each orbital within a subshell gets one electron before any orbital gets a second.
Pauli Exclusion Principle
The Pauli exclusion principle states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms). This means each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (one spin-up, one spin-down).
Determining the Ground State Electron Configuration of Sodium (Na)
Sodium (Na) has an atomic number of 11, meaning it has 11 protons and 11 electrons in its neutral state. To determine its ground state electron configuration, we follow the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
Step-by-Step Configuration
-
First Shell (n=1): The first shell contains only the 1s subshell, which can hold a maximum of two electrons. Therefore, we fill the 1s orbital with two electrons. This is represented as 1s².
-
Second Shell (n=2): The second shell contains the 2s and 2p subshells. The 2s subshell can hold two electrons, so we fill it next, resulting in 2s². The 2p subshell has three orbitals (2px, 2py, 2pz), each capable of holding two electrons, for a total of six electrons. We fill these orbitals according to Hund's rule, placing one electron in each 2p orbital before pairing them up. This gives us 2p⁶.
-
Third Shell (n=3): The third shell begins with the 3s subshell, which can hold two electrons. After filling the previous shells, we have three electrons remaining. We place these two electrons into the 3s orbital, giving us 3s¹.
Complete Electron Configuration of Sodium
Combining the filled subshells, the complete ground state electron configuration of sodium (Na) is: 1s²2s²2p⁶3s¹.
Different Notations for Electron Configuration
The electron configuration can be expressed in several ways, each offering a slightly different perspective:
-
Full Notation: This notation explicitly lists all the subshells and their electron occupancy, as shown above: 1s²2s²2p⁶3s¹.
-
Condensed Notation (Noble Gas Notation): This notation utilizes the noble gas preceding the element in the periodic table to represent the inner core electrons. For sodium, the preceding noble gas is neon (Ne), which has an electron configuration of 1s²2s²2p⁶. Therefore, the condensed notation for sodium is: [Ne]3s¹. This notation is more concise and highlights the valence electrons, which are crucial in chemical bonding.
-
Orbital Diagram: This visual representation uses boxes to represent orbitals and arrows to represent electrons. For sodium, it would show two electrons in the 1s orbital, two in 2s, six in 2p (two in each of the three 2p orbitals), and one in the 3s orbital.
Significance of Sodium's Electron Configuration
Sodium's electron configuration directly influences its properties and reactivity:
-
Valence Electron: The outermost electron in the 3s orbital is the valence electron. This single valence electron is readily lost, making sodium highly reactive and easily forming a +1 cation (Na⁺). This tendency to lose an electron is characteristic of alkali metals.
-
Reactivity: The ease with which sodium loses its valence electron explains its high reactivity. It readily reacts with nonmetals, such as chlorine (Cl), to form ionic compounds like sodium chloride (NaCl), also known as table salt. The transfer of the valence electron from sodium to chlorine results in stable octets for both ions.
-
Metallic Bonding: Sodium's metallic bonding arises from the delocalization of its valence electrons. These electrons are not tightly bound to individual sodium atoms, creating a "sea" of electrons that holds the positively charged sodium ions together. This explains sodium's characteristic metallic properties like conductivity and malleability.
-
Ionization Energy: The ionization energy, the energy required to remove an electron from an atom, is relatively low for sodium due to the loosely held valence electron.
Exceptions to the Aufbau Principle
While the Aufbau principle provides a good general guideline, there are exceptions where the predicted electron configuration based solely on the principle doesn't match the experimentally observed configuration. These exceptions primarily occur in transition metals and lanthanides/actinides due to complex electron-electron interactions and the closeness in energy levels of certain orbitals. However, sodium, being an alkali metal, follows the Aufbau principle without exception.
Applications and Relevance of Understanding Electron Configuration
Understanding electron configurations isn't merely an academic exercise. It has far-reaching implications across various fields:
-
Predicting Chemical Reactivity: Knowing the electron configuration allows us to predict how an element will react with other elements. This is fundamental in chemistry, allowing us to understand and predict chemical reactions.
-
Material Science: Electron configurations are crucial in material science, helping us design and synthesize new materials with specific properties. The electronic structure determines the material's electrical conductivity, magnetic properties, and other important characteristics.
-
Spectroscopy: The arrangement of electrons within an atom is directly related to its interaction with light. Spectroscopy utilizes this knowledge to analyze the composition of substances and study their electronic structure.
-
Nuclear Chemistry: Understanding electron configurations is essential for comprehending nuclear processes and their impact on electronic structure.
Conclusion
The ground state electron configuration of sodium, 1s²2s²2p⁶3s¹ or [Ne]3s¹, is a cornerstone concept in chemistry. Its simplicity yet profound implications provide a clear illustration of how electronic structure dictates chemical behavior. By understanding the Aufbau principle, Hund's rule, and the Pauli exclusion principle, we can predict and explain the reactivity, bonding characteristics, and other properties of sodium and other elements. This knowledge is essential for advancements in various scientific disciplines, highlighting the importance of this seemingly fundamental concept in understanding the world around us. This detailed exploration of sodium's electron configuration serves as a robust foundation for further exploration of more complex electronic structures and their chemical implications.
Latest Posts
Latest Posts
-
Are Antiparalell Beta Sheets Mrore Stable
Mar 17, 2025
-
How Do I Solve Square Root Equations
Mar 17, 2025
-
What Is A Monomer Of A Nucleic Acid
Mar 17, 2025
-
100 Most Important People Of The Century
Mar 17, 2025
-
Equations For Cellular Respiration And Photosynthesis
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Ground State Electron Configuration For Na . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
