Are Antiparalell Beta Sheets Mrore Stable
Muz Play
Mar 17, 2025 · 6 min read
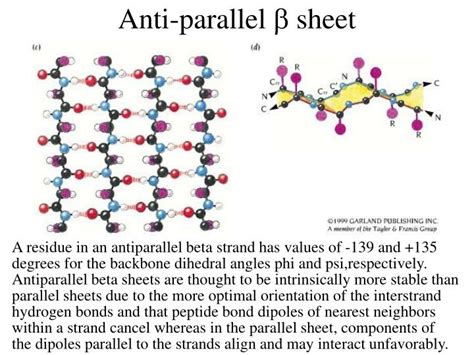
Table of Contents
Are Antiparallel Beta Sheets More Stable? A Deep Dive into Beta-Sheet Structure and Stability
Beta-sheets are fundamental secondary structures in proteins, crucial for their function and stability. Understanding their intricacies, particularly the differences between parallel and antiparallel arrangements, is key to comprehending protein folding and design. This article delves into the question of whether antiparallel beta-sheets are inherently more stable than their parallel counterparts, exploring the structural and energetic factors involved.
Understanding Beta-Sheets: A Quick Recap
Beta-sheets are formed by hydrogen bonding between backbone amide and carbonyl groups of adjacent polypeptide chains or segments of a single polypeptide chain. These chains, called beta-strands, are arranged side-by-side in a pleated sheet conformation. Crucially, these strands can be oriented in two distinct ways: parallel or antiparallel.
-
Parallel Beta-Sheets: In parallel beta-sheets, adjacent strands run in the same N-terminus to C-terminus direction. The hydrogen bonds formed between strands are angled and slightly less optimal geometrically.
-
Antiparallel Beta-Sheets: In antiparallel beta-sheets, adjacent strands run in opposite N-terminus to C-terminus directions. This arrangement allows for linear hydrogen bonds between the carbonyl group of one strand and the amide group of the opposite strand. These linear bonds are considered stronger and more energetically favorable due to the better alignment of the hydrogen bond donor and acceptor atoms.
The Energetic Argument: Why Antiparallel Often Wins
The primary reason antiparallel beta-sheets are often considered more stable stems from the energetics of hydrogen bonding. The linear hydrogen bonds in antiparallel sheets are significantly better optimized than the angled hydrogen bonds found in parallel sheets. This translates to a stronger and more stable interaction between adjacent strands.
Hydrogen Bond Geometry: The Key Difference
The near-linear arrangement of hydrogen bonds in antiparallel sheets minimizes steric hindrance and maximizes the electrostatic interaction between the donor and acceptor atoms. This near-linearity leads to stronger hydrogen bonds, contributing directly to increased stability. In contrast, the angled hydrogen bonds in parallel sheets are less efficient, resulting in weaker interactions.
Other Contributing Factors: Beyond Hydrogen Bonds
While hydrogen bonding is the dominant factor, other interactions also contribute to the stability differences between parallel and antiparallel beta-sheets.
-
Side-chain Interactions: The arrangement of side chains can influence stability. In both types of sheets, side chains alternately project above and below the plane of the sheet. However, the specific interactions between these side chains can differ depending on the amino acid sequence and the type of sheet. Favorable van der Waals interactions and hydrophobic packing can further enhance the stability of both parallel and antiparallel sheets, but the effects may vary.
-
Backbone Dihedral Angles: The backbone dihedral angles (φ and ψ) are different in parallel and antiparallel beta-sheets. These differences can subtly affect the overall energy and stability. While not as significant as hydrogen bond geometry, these variations contribute to the nuanced differences in stability.
-
Sheet Twist: Beta-sheets are not perfectly flat; they often exhibit a slight twist. This twist can be more pronounced in parallel sheets, potentially influencing their stability. The degree of twist depends on the amino acid sequence and the specific interactions within the sheet.
Exceptions to the Rule: Context Matters
It's crucial to understand that the assertion that antiparallel beta-sheets are always more stable is an oversimplification. The relative stability of parallel and antiparallel sheets depends heavily on the specific protein sequence and the overall context of the protein structure.
-
Sequence Dependence: The amino acid sequence dictates the strength of various interactions, including hydrogen bonds and side-chain interactions. A specific sequence might create particularly favorable interactions in a parallel sheet, overcoming the inherent energetic advantage of the antiparallel arrangement.
-
Environmental Factors: Factors such as pH, temperature, and the presence of ions or other molecules can influence the stability of both parallel and antiparallel beta-sheets. Changes in these environmental factors can shift the balance of stability.
-
Larger Protein Contexts: The stability of a beta-sheet isn't solely determined by its intrinsic properties. Its interactions with other secondary structures (alpha-helices, loops) and the overall tertiary structure of the protein play a critical role. A less stable beta-sheet might be essential for a specific function within the protein's overall fold.
Experimental Evidence: Studying Beta-Sheet Stability
Numerous experimental techniques have been used to study the stability of beta-sheets, providing evidence supporting the general trend of antiparallel sheets being more stable. These include:
-
X-ray crystallography: This technique reveals the precise three-dimensional structure of proteins, including the arrangement of beta-sheets. Analysis of numerous protein structures reveals a higher prevalence of antiparallel beta-sheets, indirectly supporting the notion of greater stability.
-
Nuclear magnetic resonance (NMR) spectroscopy: NMR spectroscopy provides information about the dynamics and conformation of proteins. Studies using NMR have shown that the hydrogen bonds in antiparallel sheets are generally stronger and more persistent than those in parallel sheets.
-
Molecular dynamics (MD) simulations: Computational methods like MD simulations can be used to study the stability of beta-sheets under different conditions. These simulations have consistently shown that antiparallel sheets tend to be more stable than parallel sheets, reflecting the energetic advantages discussed earlier.
-
Protein engineering experiments: Scientists manipulate protein sequences to systematically alter the arrangement of beta-sheets, observing the effects on stability. These experiments have often shown that changing a parallel sheet to an antiparallel arrangement enhances stability, again supporting the central theme.
Applications in Protein Engineering and Design
Understanding the stability differences between parallel and antiparallel beta-sheets has significant implications for protein engineering and design. This knowledge allows researchers to:
-
Design more stable proteins: By incorporating antiparallel beta-sheets strategically, researchers can enhance the stability of engineered proteins, making them more robust and functional.
-
Predict protein structure: The relative stability of different beta-sheet arrangements can be incorporated into algorithms that predict protein structure from amino acid sequence.
-
Develop novel therapeutic proteins: The design of therapeutic proteins with enhanced stability and longer half-lives benefits greatly from a deep understanding of beta-sheet stability.
Conclusion: A Nuance to the Narrative
While the general consensus points towards antiparallel beta-sheets exhibiting greater stability due to the optimized geometry of their hydrogen bonds, it is critical to emphasize the influence of context. The specific amino acid sequence, surrounding environment, and the protein's overall three-dimensional structure all significantly contribute to the relative stability of both parallel and antiparallel arrangements. The energetic preference for antiparallel sheets provides a valuable guideline, but exceptions exist, highlighting the complexity and beauty of protein structure and function. Further research continues to refine our understanding of these nuanced interactions, pushing the boundaries of protein engineering and our ability to design proteins with specific properties. The exploration of beta-sheet stability remains a vibrant and important area of research with far-reaching implications for various scientific disciplines.
Latest Posts
Latest Posts
-
Enzymes Decrease The Activation Energy Of A Reaction By
Mar 17, 2025
-
Does Water Go From High To Low Concentration
Mar 17, 2025
-
Circle Math Triangle Extending From Circle
Mar 17, 2025
-
According To The Kinetic Theory Of Gases
Mar 17, 2025
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Are Antiparalell Beta Sheets Mrore Stable . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
