Enzymes Decrease The Activation Energy Of A Reaction By
Muz Play
Mar 17, 2025 · 5 min read
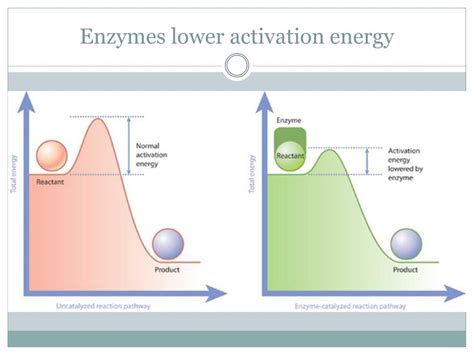
Table of Contents
Enzymes Decrease the Activation Energy of a Reaction By… Clever Mechanisms!
Enzymes are biological catalysts, remarkable molecules that accelerate the rate of virtually all chemical reactions within living organisms. They achieve this feat by significantly lowering the activation energy of a reaction, the energy barrier that must be overcome for reactants to transform into products. Understanding how enzymes achieve this reduction is crucial to comprehending the fundamental processes of life. This article delves deep into the intricate mechanisms by which enzymes decrease activation energy, exploring the key players and the elegant choreography of molecular interactions.
The Activation Energy Hurdle: A Biological Speed Bump
Before diving into the enzyme-driven solutions, let's revisit the concept of activation energy. Every chemical reaction, whether it's the digestion of food or the replication of DNA, requires a certain amount of energy to initiate. This energy is the activation energy (Ea). Think of it as a hill a molecule must climb before it can roll down to the product side. Without sufficient energy, the reaction remains stalled.
The higher the activation energy, the slower the reaction rate. Many biologically important reactions have high activation energies, meaning they'd proceed extremely slowly without enzymatic intervention. Enzymes, therefore, are essential for life because they drastically reduce this activation energy barrier, allowing reactions to proceed at rates compatible with life's needs.
How Enzymes Lower Activation Energy: A Multifaceted Approach
Enzymes accomplish this remarkable feat through several sophisticated mechanisms, often working in concert:
1. Substrate Binding and Orientation: Bringing Reactants Together
One of the primary ways enzymes lower activation energy is by precisely binding their substrates (the molecules they act upon). The enzyme's active site, a specifically shaped region, binds substrates with remarkable specificity. This binding brings the reactants into close proximity and in the optimal orientation required for the reaction to occur. Without the enzyme, the substrates would collide randomly, and the likelihood of a successful reaction would be significantly lower. This effect is akin to arranging bowling pins in a precise formation for an optimal strike, rather than throwing the ball at a randomly scattered arrangement.
2. Induced Fit: A Dynamic Embrace
The interaction between the enzyme and substrate isn't static; it's dynamic. The "induced fit" model proposes that the binding of the substrate induces a conformational change in the enzyme, further optimizing the active site for catalysis. This change can involve subtle shifts in amino acid residues, bringing catalytic groups into closer proximity to the reactive centers of the substrates. This precise molding of the active site around the substrate ensures the optimal arrangement for reaction, much like a tailor carefully adjusting a garment for a perfect fit.
3. Stabilization of the Transition State: Lowering the Energy Peak
The highest-energy point along the reaction coordinate is called the transition state. This is the point where the bonds in the reactants are breaking and new bonds are forming. Enzymes dramatically lower the energy of this transition state. They achieve this by stabilizing the transition state through a variety of mechanisms, including:
- Electrostatic Interactions: Charged amino acid residues in the active site can interact with the substrates, stabilizing the transition state through electrostatic attractions or repulsions.
- Hydrogen Bonding: Hydrogen bonds between enzyme and substrate can also contribute to the stabilization of the transition state.
- Hydrophobic Interactions: Nonpolar regions of the enzyme can interact with hydrophobic regions of the substrates, facilitating the proper orientation and reducing the energy required for the reaction to proceed.
This stabilization of the transition state is analogous to building scaffolding around a construction site to provide support and reduce the energy required for building the structure.
4. Acid-Base Catalysis: Proton Transfers for Efficiency
Many enzymatic reactions involve the transfer of protons (H+ ions). Enzymes often utilize specific amino acid residues in their active sites to act as acids or bases, facilitating these proton transfers. Acidic residues donate protons, while basic residues accept them. These precise proton transfers are crucial for stabilizing charged intermediates in the reaction pathway, dramatically reducing the activation energy. This is similar to using specific tools – acids and bases – to precisely manipulate the construction materials during building.
5. Covalent Catalysis: Temporary Bonds for Reaction Enhancement
In some cases, enzymes utilize covalent catalysis, forming a transient covalent bond between the enzyme and the substrate. This bond creates a new reaction pathway with a lower activation energy. Once the reaction is complete, the enzyme returns to its original state, ready to catalyze another reaction. This mechanism is like creating a temporary bridge to shorten the distance between two points, thus reducing the energy required for transit.
6. Metal Ion Catalysis: Ionic Interactions for Stability
Metal ions, such as zinc, magnesium, and iron, are frequently found in enzyme active sites. These ions participate in catalysis in various ways. They can act as:
- Lewis acids: Accepting electron pairs from substrates, thereby polarizing bonds and making them more reactive.
- Electrostatic stabilizers: Stabilizing negatively charged intermediates in the reaction.
- Redox agents: Participating in electron transfer reactions.
The metal ion acts as an essential helper, much like a skilled assistant aiding the main worker in completing a task efficiently.
Specificity and Efficiency: The hallmarks of Enzymatic Action
The remarkable efficiency of enzymes stems not only from their ability to lower activation energy but also from their high degree of specificity. Each enzyme is highly selective for its substrates, ensuring that the correct reaction occurs. This specificity is crucial for maintaining the intricate balance of biochemical reactions within living cells. The precision is analogous to a perfectly designed key fitting into its specific lock – other keys won’t work.
Conclusion: A Symphony of Molecular Interactions
In conclusion, enzymes decrease the activation energy of reactions through a sophisticated interplay of mechanisms. They precisely bind substrates, induce conformational changes, stabilize transition states, utilize acid-base and covalent catalysis, and often employ metal ions to achieve remarkable catalytic efficiency. This intricate molecular dance is essential for life, allowing biological reactions to proceed at rates compatible with the needs of living organisms. Further research continues to unveil the intricacies of enzyme mechanisms, providing deeper insights into the remarkable efficiency and specificity of these biological workhorses. The detailed understanding of these mechanisms is not only of fundamental scientific importance but also has implications for drug design, industrial biotechnology, and other applications. Enzymes are more than just catalysts; they are the intricate engines that drive the processes of life.
Latest Posts
Latest Posts
-
What Are The Three Parameters Of Hypergeometric Pmfs
Mar 17, 2025
-
Difference Between Column And Thin Layer Chromatography
Mar 17, 2025
-
Opening A 6 Membered Ring Mechanism
Mar 17, 2025
-
What Is The Base Of A Triangle
Mar 17, 2025
-
Electrons Are Located In Energy Levels Called Electron
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Enzymes Decrease The Activation Energy Of A Reaction By . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
