Heat Capacity And Specific Heat Problems
Muz Play
Mar 20, 2025 · 6 min read
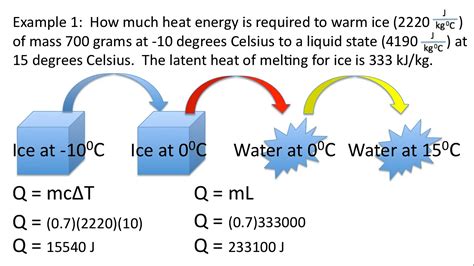
Table of Contents
Heat Capacity and Specific Heat: Problems and Solutions
Understanding heat capacity and specific heat is crucial in various fields, from thermodynamics and material science to engineering and meteorology. These concepts describe how substances respond to changes in temperature when heat is added or removed. This comprehensive guide delves into the intricacies of heat capacity and specific heat, providing a thorough explanation of the concepts, common problems encountered, and step-by-step solutions to help you master this important topic.
What is Heat Capacity?
Heat capacity (C) is a measure of a substance's ability to absorb heat energy. It quantifies the amount of heat required to raise the temperature of a substance by one degree Celsius (or one Kelvin). The formula for heat capacity is:
C = Q / ΔT
Where:
- C represents the heat capacity (measured in Joules per Kelvin, J/K, or Joules per degree Celsius, J/°C).
- Q represents the amount of heat energy transferred (measured in Joules, J).
- ΔT represents the change in temperature (measured in Kelvin, K, or degrees Celsius, °C).
It's important to note that the heat capacity of a substance is dependent on its mass. A larger mass requires more heat to achieve the same temperature change. This leads us to the concept of specific heat.
What is Specific Heat?
Specific heat (c) is a more refined measure, representing the amount of heat required to raise the temperature of one gram (or one kilogram) of a substance by one degree Celsius (or one Kelvin). It's an intensive property, meaning it doesn't depend on the amount of substance present. The formula for specific heat is:
c = Q / (mΔT)
Where:
- c represents the specific heat (measured in Joules per gram-Kelvin, J/g·K, or Joules per kilogram-Kelvin, J/kg·K).
- Q represents the amount of heat energy transferred (measured in Joules, J).
- m represents the mass of the substance (measured in grams, g, or kilograms, kg).
- ΔT represents the change in temperature (measured in Kelvin, K, or degrees Celsius, °C).
Specific heat is a fundamental property of a material and varies significantly between substances. Water, for instance, has a remarkably high specific heat compared to many other materials. This high specific heat makes water an excellent heat reservoir and plays a crucial role in regulating Earth's climate.
Relationship Between Heat Capacity and Specific Heat
Heat capacity and specific heat are closely related. The heat capacity (C) of a substance is simply the product of its specific heat (c) and its mass (m):
C = mc
This equation highlights the relationship between the extensive property (heat capacity) and the intensive property (specific heat).
Common Problems Involving Heat Capacity and Specific Heat
Many problems involving heat capacity and specific heat require the application of the formulas mentioned above, often combined with other thermodynamic principles. Here are some common problem types:
Problem Type 1: Calculating Heat Capacity or Specific Heat
Problem: A sample of metal weighing 50 grams absorbs 1000 J of heat, causing its temperature to increase by 20°C. Calculate the specific heat of the metal.
Solution:
- Identify the knowns: m = 50 g, Q = 1000 J, ΔT = 20°C
- Apply the specific heat formula: c = Q / (mΔT)
- Substitute the values: c = 1000 J / (50 g * 20°C) = 1 J/g·°C
Therefore, the specific heat of the metal is 1 J/g·°C.
Problem Type 2: Calculating Heat Transfer
Problem: 200 grams of water at 25°C absorbs 10,000 J of heat. Assuming the specific heat of water is 4.18 J/g·°C, what is the final temperature of the water?
Solution:
- Identify the knowns: m = 200 g, Q = 10000 J, c = 4.18 J/g·°C, Tᵢ = 25°C
- Rearrange the specific heat formula to solve for ΔT: ΔT = Q / (mc)
- Substitute the values: ΔT = 10000 J / (200 g * 4.18 J/g·°C) ≈ 11.96°C
- Calculate the final temperature: T<sub>f</sub> = Tᵢ + ΔT = 25°C + 11.96°C ≈ 36.96°C
The final temperature of the water is approximately 36.96°C.
Problem Type 3: Problems Involving Multiple Substances
Problem: 100 g of aluminum (c = 0.9 J/g·°C) at 100°C is added to 200 g of water (c = 4.18 J/g·°C) at 20°C. Assuming no heat is lost to the surroundings, what is the final equilibrium temperature?
Solution:
This problem involves heat transfer between two substances. The heat lost by the aluminum is equal to the heat gained by the water.
- Set up the heat transfer equation: Q<sub>aluminum</sub> = -Q<sub>water</sub>
- Express heat transfer using the specific heat formula: m<sub>aluminum</sub>c<sub>aluminum</sub>ΔT<sub>aluminum</sub> = -m<sub>water</sub>c<sub>water</sub>ΔT<sub>water</sub>
- Substitute the known values: (100 g)(0.9 J/g·°C)(T<sub>f</sub> - 100°C) = -(200 g)(4.18 J/g·°C)(T<sub>f</sub> - 20°C)
- Solve for T<sub>f</sub>: This is a simple algebraic equation that can be solved for the final temperature, T<sub>f</sub>. (Note: The solution requires careful algebraic manipulation).
Problem Type 4: Calorimetry Problems
Calorimetry problems involve using a calorimeter, a device designed to measure heat transfer. These problems often involve calculating the specific heat of an unknown substance by observing its effect on the temperature of a known substance within the calorimeter. These problems generally involve a similar approach as Problem Type 3, but with additional considerations for the calorimeter's heat capacity.
Problem Type 5: Phase Change Problems
Phase changes (e.g., melting, boiling) involve additional heat transfer beyond simple temperature changes. The heat required for a phase change is given by:
Q = mL
Where:
- Q is the heat transferred.
- m is the mass of the substance.
- L is the latent heat (heat of fusion for melting/freezing, heat of vaporization for boiling/condensation).
Problems involving phase changes require incorporating this equation alongside the specific heat equation. For example, calculating the total heat needed to raise the temperature of ice from -10°C to steam at 120°C would require considering the heat needed to warm the ice, melt the ice, warm the water, boil the water, and finally warm the steam.
Advanced Concepts and Applications
The concepts of heat capacity and specific heat have numerous advanced applications:
- Material Selection: Engineers consider specific heat when selecting materials for applications requiring heat transfer control (e.g., heat sinks, insulators).
- Climate Modeling: Specific heat of water is crucial in understanding climate patterns and weather forecasting.
- Chemical Reactions: Heat capacity plays a role in understanding the energetics of chemical reactions.
- Phase Diagrams: Specific heat contributes to the construction and interpretation of phase diagrams, which show the relationship between temperature, pressure, and the phases of a substance.
Conclusion
Understanding heat capacity and specific heat is essential for mastering thermodynamics and related fields. By applying the fundamental formulas and carefully analyzing the problems, one can effectively solve a wide range of heat transfer problems, from simple calculations to more complex scenarios involving multiple substances and phase changes. Remember to carefully identify the known variables, utilize the appropriate formulas, and solve the equations systematically. With practice and a solid grasp of the underlying principles, you can confidently tackle any heat capacity and specific heat problem that comes your way. This comprehensive guide provides a solid foundation for further exploration into this critical area of physics and engineering.
Latest Posts
Latest Posts
-
How To Simplify Radicals In A Fraction
Mar 21, 2025
-
A Primary Reinforcer For A Person Would Be
Mar 21, 2025
-
Cells Are The Basic Unit Of
Mar 21, 2025
-
Is Carbon Dioxide A Pure Substance
Mar 21, 2025
-
Map Of North Africa Southwest Asia
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Heat Capacity And Specific Heat Problems . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
