High Spin And Low Spin Complexes
Muz Play
Mar 15, 2025 · 7 min read
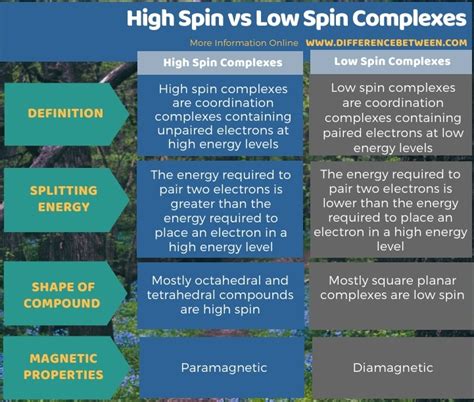
Table of Contents
High Spin and Low Spin Complexes: A Deep Dive into Coordination Chemistry
Coordination complexes, the fascinating realm where metal ions bond with ligands, exhibit a rich diversity in their properties. One crucial aspect governing this diversity is the spin state of the central metal ion, leading to the classification of complexes as high spin or low spin. Understanding the factors that determine high spin versus low spin configurations is key to comprehending the reactivity, magnetism, and spectroscopic properties of these compounds. This article delves into the intricacies of high spin and low spin complexes, exploring the underlying principles and their implications.
Understanding Crystal Field Theory (CFT) and Ligand Field Theory (LFT)
Before we embark on the specifics of high spin and low spin complexes, it's crucial to establish a foundational understanding of the theoretical frameworks that explain their existence: Crystal Field Theory (CFT) and Ligand Field Theory (LFT).
Crystal Field Theory (CFT): A Simplified Model
CFT provides a relatively simple model to understand the splitting of d-orbitals in transition metal complexes. It treats ligands as point negative charges that repel the d-electrons on the central metal ion. When ligands approach the metal ion, the degeneracy of the five d-orbitals is lifted. In an octahedral complex, for instance, the d-orbitals split into two sets:
- t<sub>2g</sub> set: Three lower-energy orbitals (d<sub>xy</sub>, d<sub>xz</sub>, d<sub>yz</sub>)
- e<sub>g</sub> set: Two higher-energy orbitals (d<sub>z²</sub>, d<sub>x²-y²</sub>)
The energy difference between these two sets is denoted by Δ<sub>o</sub> (the octahedral crystal field splitting parameter). The magnitude of Δ<sub>o</sub> is crucial in determining whether a complex will be high spin or low spin.
Ligand Field Theory (LFT): A More Refined Approach
While CFT provides a useful qualitative picture, it doesn't fully account for the covalent nature of metal-ligand bonding. Ligand Field Theory (LFT) addresses this limitation by incorporating molecular orbital theory. LFT considers the overlap of metal d-orbitals with ligand orbitals, leading to the formation of bonding and antibonding molecular orbitals. The energy difference between these orbitals is analogous to Δ<sub>o</sub> in CFT, but it reflects the covalent interactions as well.
The Role of Δ<sub>o</sub> and Pairing Energy (P)
The crucial factor determining whether a complex is high spin or low spin is the relative magnitudes of Δ<sub>o</sub> (or the analogous energy splitting in LFT) and the pairing energy (P). Pairing energy represents the energy required to pair two electrons in the same orbital. This energy arises from electron-electron repulsion.
-
High Spin Complexes: When Δ<sub>o</sub> < P, it's energetically more favorable for electrons to occupy each of the d-orbitals individually before pairing up. This leads to a maximum number of unpaired electrons, resulting in a high spin complex.
-
Low Spin Complexes: When Δ<sub>o</sub> > P, it's energetically more favorable to pair electrons in the lower energy t<sub>2g</sub> orbitals before occupying the higher energy e<sub>g</sub> orbitals. This leads to a minimum number of unpaired electrons, resulting in a low spin complex.
Factors Influencing Δ<sub>o</sub> and Spin State
Several factors influence the magnitude of Δ<sub>o</sub> and consequently the spin state of a complex:
1. The Nature of the Ligand: The Spectrochemical Series
Ligands are arranged in a spectrochemical series based on their ability to increase Δ<sub>o</sub>. Strong-field ligands, such as CN⁻ and CO, cause a large splitting of the d-orbitals, favoring low spin complexes. Weak-field ligands, such as I⁻ and Br⁻, cause a smaller splitting, favoring high spin complexes. The spectrochemical series is as follows (from weak-field to strong-field ligands):
I⁻ < Br⁻ < S²⁻ < SCN⁻ < Cl⁻ < NO₃⁻ < N₃⁻ < F⁻ < OH⁻ < C₂O₄²⁻ < H₂O < NCS⁻ < CH₃CN < py < NH₃ < en < bipy < phen < NO₂⁻ < PPh₃ < CN⁻ < CO
2. The Oxidation State of the Metal Ion
The oxidation state of the metal ion significantly influences Δ<sub>o</sub>. Higher oxidation states generally lead to larger Δ<sub>o</sub> values because the increased positive charge on the metal ion attracts the electrons from the ligands more strongly, enhancing the ligand field.
3. The Geometry of the Complex
The geometry of the complex also plays a role. Octahedral complexes are the most common, but other geometries like tetrahedral and square planar complexes also exhibit d-orbital splitting. The magnitude of the splitting varies with geometry, influencing the spin state. Tetrahedral complexes, for example, generally have smaller splitting than octahedral complexes, favoring high spin configurations.
4. The Metal Ion
The identity of the metal ion itself affects Δ<sub>o</sub>. Different metal ions have different effective nuclear charges and d-electron configurations, influencing the interaction with ligands and, hence, the magnitude of the splitting.
Magnetic Properties: A Key Distinguishing Feature
High spin and low spin complexes exhibit distinct magnetic properties. High spin complexes possess a larger number of unpaired electrons, leading to paramagnetism, a phenomenon where the complex is attracted to a magnetic field. Low spin complexes, having fewer or no unpaired electrons, exhibit diamagnetism, meaning they are slightly repelled by a magnetic field. Magnetic susceptibility measurements are a crucial technique for determining the spin state of a coordination complex.
Spectroscopic Techniques: Unveiling Electronic Structure
Various spectroscopic techniques provide invaluable insights into the electronic structure and spin state of complexes.
-
UV-Vis Spectroscopy: The electronic transitions between the d-orbitals are responsible for the characteristic colors of transition metal complexes. The absorption spectra reveal information about the energy difference between the d-orbitals (Δ<sub>o</sub>), providing clues about the ligand field strength and spin state.
-
Electron Paramagnetic Resonance (EPR) Spectroscopy: EPR is a powerful technique specifically used to study paramagnetic species. It directly probes the unpaired electrons, providing detailed information about their number, spin, and environment, thus confirming the high spin nature of the complex.
Examples of High Spin and Low Spin Complexes
Let's consider some specific examples to illustrate the concepts discussed:
-
[Fe(H₂O)₆]²⁺ (High Spin): Fe²⁺ is a d⁶ ion. Water is a weak-field ligand. Therefore, Δ<sub>o</sub> < P, resulting in a high spin configuration with four unpaired electrons.
-
[Fe(CN)₆]⁴⁻ (Low Spin): Fe²⁺ is again a d⁶ ion, but cyanide (CN⁻) is a strong-field ligand. Here, Δ<sub>o</sub> > P, leading to a low spin configuration with no unpaired electrons.
-
[Co(NH₃)₆]³⁺ (Low Spin): Co³⁺ is a d⁶ ion, and ammonia (NH₃) is a relatively strong-field ligand. This results in a low spin configuration with no unpaired electrons.
-
[CoF₆]³⁻ (High Spin): Co³⁺ is a d⁶ ion, but fluoride (F⁻) is a weak-field ligand. This leads to a high spin configuration with four unpaired electrons.
Applications and Significance
Understanding the spin state of coordination complexes is critical in various fields:
-
Catalysis: The spin state influences the reactivity of metal complexes, making them suitable catalysts for specific reactions.
-
Materials Science: The magnetic properties of high spin and low spin complexes are exploited in the development of magnetic materials.
-
Bioinorganic Chemistry: Many metalloenzymes rely on the specific spin state of the metal ion for their biological function. Hemoglobin, for example, exhibits a spin state change upon oxygen binding.
-
Medicine: Metal complexes are used in various medicinal applications, and their spin state can influence their therapeutic efficacy and toxicity.
Conclusion
The distinction between high spin and low spin complexes is a cornerstone concept in coordination chemistry. It hinges on the interplay between the crystal field splitting energy (Δ<sub>o</sub>) and the electron pairing energy (P). Factors like the nature of the ligand, the oxidation state of the metal ion, the geometry of the complex, and the identity of the metal ion all contribute to determining the spin state. This understanding is crucial for comprehending the diverse properties of coordination complexes and their numerous applications across various scientific disciplines. Further research continues to expand our knowledge in this fascinating field, unveiling new applications and deeper insights into the intricate relationships between electronic structure, spin state, and complex behavior.
Latest Posts
Latest Posts
-
Boiling Point On Graph In Celsius
Mar 15, 2025
-
List The Classification Levels From Broadest To Most Specific
Mar 15, 2025
-
Equipments For Measuring Volume Of Acids
Mar 15, 2025
-
The Acid Test Tells Whether A Mineral Is Called
Mar 15, 2025
-
Definition Contour Integral Union Of Curves
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about High Spin And Low Spin Complexes . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
