How Are Gases Different From Solids And Liquids
Muz Play
Mar 15, 2025 · 6 min read
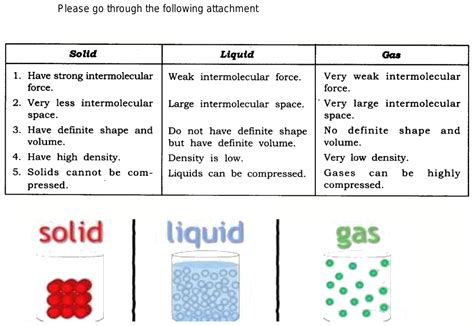
Table of Contents
How Are Gases Different from Solids and Liquids? A Deep Dive into States of Matter
Understanding the differences between solids, liquids, and gases is fundamental to comprehending the physical world around us. While all three are states of matter composed of atoms and molecules, their distinct properties stem from the varying degrees of freedom and interactions between these particles. This comprehensive guide explores the key differences between gases and their condensed counterparts, solids and liquids, delving into their microscopic structure, macroscopic behavior, and the transitions between states.
Microscopic Structure: The Key to Understanding Differences
The fundamental difference lies in the arrangement and movement of particles at the microscopic level. This microscopic structure dictates the macroscopic properties we observe.
Solids: Order and Structure
In solids, particles (atoms, ions, or molecules) are tightly packed in a highly ordered, three-dimensional arrangement. These particles are held together by strong intermolecular forces, which restrict their movement to vibrations around fixed positions. This close packing gives solids their characteristic rigidity and definite shape and volume. The type of bonding – ionic, covalent, metallic – significantly influences the properties of the solid. For instance, ionic solids tend to be brittle due to the strong electrostatic forces between ions, while metallic solids are often malleable and ductile due to the delocalized electrons.
Liquids: Moderate Order and Freedom of Movement
Liquids possess a less ordered structure than solids. While particles are still relatively close together, their arrangement is less regular and more fluid. Intermolecular forces are weaker than in solids, allowing particles more freedom of movement. They can slide past one another, leading to liquids' ability to flow and conform to the shape of their container. However, liquids maintain a definite volume because intermolecular forces still prevent particles from completely separating.
Gases: Chaos and Independence
Gases stand in stark contrast to solids and liquids. Particles in a gas are widely dispersed, with large distances separating them. Intermolecular forces are extremely weak, almost negligible in comparison to solids and liquids. This allows gas particles to move freely and independently, exhibiting random motion in all directions. Consequently, gases have neither a definite shape nor a definite volume; they expand to fill the container they occupy.
Macroscopic Properties: Observing the Differences
The differences in microscopic structure translate directly into observable macroscopic properties. Let's examine these key differences in detail:
1. Shape and Volume: A Defining Characteristic
- Solids: Possess a definite shape and volume. They resist compression and maintain their shape and volume regardless of the container.
- Liquids: Have a definite volume but take the shape of their container. They are relatively incompressible, meaning their volume changes minimally under pressure.
- Gases: Have neither a definite shape nor a definite volume. They expand to fill the entire available space and are highly compressible, meaning their volume can be significantly reduced by applying pressure.
2. Density: Packing and Mass
- Solids: Generally have the highest density of the three states due to the close packing of particles.
- Liquids: Have a lower density than solids but a higher density than gases.
- Gases: Have the lowest density because particles are widely dispersed.
3. Compressibility: Responding to Pressure
- Solids: Are largely incompressible; applying pressure has little effect on their volume.
- Liquids: Are relatively incompressible, exhibiting minimal changes in volume under pressure.
- Gases: Are highly compressible; applying pressure significantly reduces their volume as particles are forced closer together.
4. Diffusion and Effusion: Particle Movement
- Solids: Diffusion is extremely slow in solids due to the restricted particle movement.
- Liquids: Diffusion occurs at a moderate rate as particles can move and interact.
- Gases: Diffusion and effusion (the escape of gas through a small opening) are rapid due to the high particle mobility and weak intermolecular forces.
5. Expansion and Contraction: Responding to Temperature
- Solids: Expand slightly upon heating and contract upon cooling due to changes in the vibrational energy of particles.
- Liquids: Expand and contract more significantly than solids upon changes in temperature.
- Gases: Exhibit the greatest expansion and contraction with temperature changes due to the significant impact of temperature on particle kinetic energy.
Phase Transitions: Shifting Between States
The three states of matter are not immutable; they can transition between each other through changes in temperature and pressure. These transitions involve energy changes, with energy being absorbed during phase transitions that increase the freedom of particle movement (e.g., melting, vaporization, sublimation) and energy being released during phase transitions that decrease the freedom of movement (e.g., freezing, condensation, deposition).
Key Phase Transitions:
- Melting: Solid to liquid (requires energy)
- Freezing: Liquid to solid (releases energy)
- Vaporization (Boiling/Evaporation): Liquid to gas (requires energy)
- Condensation: Gas to liquid (releases energy)
- Sublimation: Solid to gas (requires energy)
- Deposition: Gas to solid (releases energy)
Understanding these phase transitions is crucial in many applications, from industrial processes to weather forecasting. The conditions under which these transitions occur depend on the intermolecular forces and the substance's properties.
Real-World Applications: The Significance of Understanding States of Matter
The differences between solids, liquids, and gases have profound implications for numerous real-world applications across diverse fields.
Engineering and Material Science:
Choosing the appropriate material for a specific application hinges on its state of matter and its properties. The strength and rigidity of solids are crucial in construction, while the fluidity of liquids is essential in hydraulic systems. Gases play critical roles in various applications, including pneumatic systems and aerospace engineering.
Chemistry and Chemical Processes:
Understanding the states of matter is essential in various chemical reactions and processes. The rate of reaction, equilibrium, and solubility are all influenced by the state of the reactants and products. Gas laws, for instance, are fundamental in designing chemical reactors and predicting reaction yields.
Meteorology and Climate Science:
The behavior of gases, particularly water vapor in the atmosphere, determines weather patterns and climate conditions. The transitions between water's three states – solid (ice), liquid (water), and gas (water vapor) – drive weather phenomena like rain, snow, and cloud formation.
Biology and Medicine:
Understanding states of matter is crucial in various biological processes. The properties of water in its three states are fundamental to life, playing a vital role in temperature regulation and various biological functions. In medicine, gases like oxygen are essential for respiration, and the properties of liquids and solids determine drug delivery and tissue engineering applications.
Conclusion: A Deeper Understanding of Our World
The differences between solids, liquids, and gases arise from the fundamental differences in the arrangement and movement of their constituent particles. This microscopic structure dictates the macroscopic properties we observe, from shape and volume to density and compressibility. Understanding these differences is crucial for interpreting the physical world, driving technological advancements, and addressing critical challenges in various scientific and engineering disciplines. This comprehensive analysis serves as a stepping stone towards a deeper appreciation of the diverse and fascinating world of matter.
Latest Posts
Latest Posts
-
Increased Tympanic Membrane Flexibility Older Adults
Mar 15, 2025
-
How To Calculate The Density Of A Rock
Mar 15, 2025
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 15, 2025
-
How Many Unpaired Electrons Are In Sulfur Atom
Mar 15, 2025
-
Similarities Between The Romanticism And Transcendentalism Movement
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Are Gases Different From Solids And Liquids . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
