How Many Unpaired Electrons Are In Sulfur Atom
Muz Play
Mar 15, 2025 · 5 min read
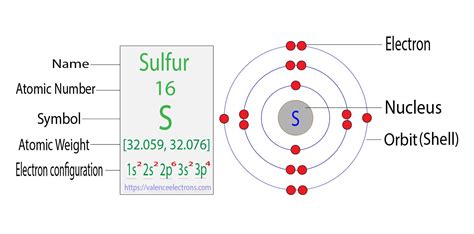
Table of Contents
How Many Unpaired Electrons Are in a Sulfur Atom? A Deep Dive into Atomic Structure and Electron Configuration
Understanding the number of unpaired electrons in a sulfur atom is fundamental to grasping its chemical behavior and properties. This seemingly simple question opens the door to a fascinating exploration of atomic structure, electron configuration, Hund's rule, and the implications for sulfur's reactivity. Let's delve into the details.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle unpaired electrons, let's refresh our understanding of the basic building blocks of an atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; sulfur has 16 protons.
- Neutrons: Neutral particles also residing in the nucleus. The number of neutrons can vary within an element (isotopes), but it doesn't affect the chemical properties.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons determine an atom's chemical behavior and reactivity.
Electron Configuration and Orbitals
Electrons occupy specific regions around the nucleus called orbitals. These orbitals are grouped into shells and subshells. The electron configuration describes the arrangement of electrons within these shells and subshells. It follows specific rules:
- Aufbau Principle: Electrons fill the lowest energy levels first.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins (represented as ↑ and ↓).
- Hund's Rule: Within a subshell, electrons will occupy individual orbitals singly before pairing up. This maximizes the total spin and minimizes electron-electron repulsion.
Sulfur's Electron Configuration: Unveiling the Mystery
Sulfur (S) has an atomic number of 16, meaning it has 16 electrons. Following the Aufbau principle and Hund's rule, the electron configuration of sulfur is: 1s²2s²2p⁶3s²3p⁴.
Let's break this down:
- 1s²: Two electrons in the 1s orbital (the lowest energy level).
- 2s²: Two electrons in the 2s orbital.
- 2p⁶: Six electrons in the 2p subshell (three orbitals, each with two electrons).
- 3s²: Two electrons in the 3s orbital.
- 3p⁴: Four electrons in the 3p subshell (three orbitals).
This is where things get interesting regarding unpaired electrons.
Applying Hund's Rule to Sulfur's 3p Subshell
The 3p subshell has three orbitals (3px, 3py, 3pz). According to Hund's rule, the four electrons in the 3p subshell will fill these orbitals individually before pairing up. This results in:
- One orbital with two paired electrons (↑↓).
- Two orbitals each with one unpaired electron (↑).
Therefore, sulfur has two unpaired electrons.
Visualizing Sulfur's Electron Configuration
To further clarify, consider a visual representation:
3p: ↑ ↑ ↑ _
The underscores represent empty orbitals. Notice that two electrons are unpaired. This configuration significantly impacts sulfur's chemical properties.
The Significance of Unpaired Electrons: Implications for Chemical Reactivity
The presence of unpaired electrons directly influences an element's reactivity. Unpaired electrons are highly reactive because they can readily form bonds with other atoms to achieve a more stable electron configuration, often following the octet rule (eight electrons in the outermost shell).
Sulfur's two unpaired electrons make it relatively reactive. It readily forms covalent bonds with other elements, sharing its unpaired electrons to complete its octet. This explains why sulfur participates in numerous chemical reactions and forms a variety of compounds.
Sulfur's Chemical Behavior: A Consequence of Unpaired Electrons
The presence of unpaired electrons in sulfur directly affects its chemical behavior in several ways:
- Covalent Bonding: Sulfur readily forms covalent bonds with other nonmetals by sharing its unpaired electrons. Examples include sulfur dioxide (SO2), sulfur trioxide (SO3), and hydrogen sulfide (H2S).
- Oxidation States: Sulfur exhibits various oxidation states, reflecting its ability to gain or lose electrons to achieve a stable configuration. The presence of unpaired electrons contributes to this versatility.
- Formation of Polyatomic Ions: Sulfur forms various polyatomic ions, such as sulfate (SO₄²⁻) and sulfite (SO₃²⁻), where the unpaired electrons participate in bonding with oxygen atoms.
- Formation of allotropes: Different arrangements of sulfur atoms can lead to different allotropes, such as rhombic sulfur and monoclinic sulfur, influencing its physical and chemical properties. The underlying electronic structure remains the same, however.
Beyond Sulfur: Unpaired Electrons in Other Elements
The concept of unpaired electrons and their influence on reactivity extends beyond sulfur. Many other elements have unpaired electrons, leading to a diverse range of chemical behaviors and compound formations. For example, oxygen (O) with two unpaired electrons is highly reactive, while the noble gases with filled electron shells and no unpaired electrons are remarkably inert.
Determining Unpaired Electrons: Methods and Techniques
While we've discussed the conceptual approach using electron configuration and Hund's rule, determining the number of unpaired electrons in an atom experimentally involves techniques like:
- Electron Spin Resonance (ESR) Spectroscopy: This technique directly measures the magnetic moment arising from unpaired electrons, providing information about their number and environment.
- Magnetic Susceptibility Measurements: Measuring the degree to which a substance is attracted or repelled by a magnetic field can indicate the presence and number of unpaired electrons.
Conclusion: The Importance of Unpaired Electrons in Chemistry
The number of unpaired electrons in an atom, as exemplified by sulfur's two unpaired electrons, is a crucial factor determining its chemical properties and reactivity. Understanding electron configuration, Hund's rule, and the implications of unpaired electrons provides a deeper appreciation for the intricacies of chemical bonding and the remarkable diversity of chemical compounds. This knowledge is essential in various fields, including material science, biochemistry, and environmental chemistry. The seemingly simple question of how many unpaired electrons are in a sulfur atom unlocks a vast world of chemical understanding.
Latest Posts
Latest Posts
-
Molecular Nature Of Matter And Change
Mar 15, 2025
-
What Is A Property Of An Element
Mar 15, 2025
-
Which State Of Matter Has No Definite Shape Or Volume
Mar 15, 2025
-
A Starting Material In A Chemical Reaction
Mar 15, 2025
-
A Solution That Contains The Maximum Amount Of Solute
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Unpaired Electrons Are In Sulfur Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
