A Solution That Contains The Maximum Amount Of Solute
Muz Play
Mar 15, 2025 · 5 min read
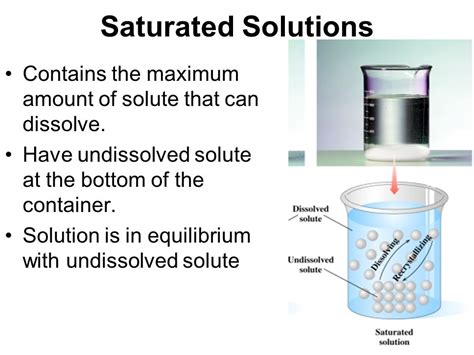
Table of Contents
A Solution That Contains the Maximum Amount of Solute: Understanding Saturation
Understanding solutions is fundamental to various scientific disciplines, from chemistry and biology to environmental science and medicine. A solution, at its core, is a homogeneous mixture of two or more substances. One component, the solute, is dissolved in another component, the solvent. The ability of a solvent to dissolve a solute is crucial, and a key concept here is saturation. This article delves deep into the concept of a solution containing the maximum amount of solute, exploring the factors that influence saturation, its implications, and practical applications.
What is a Saturated Solution?
A saturated solution is a solution that contains the maximum amount of solute that can be dissolved in a given amount of solvent at a specific temperature and pressure. Adding more solute to a saturated solution will not result in further dissolution; the excess solute will simply remain undissolved at the bottom of the container. This is a state of dynamic equilibrium, where the rate of dissolution of the solute equals the rate of crystallization (precipitation) of the solute from the solution.
Think of it like filling a glass with sugar and water. Initially, you can dissolve a significant amount of sugar. But as you keep adding more sugar, a point is reached where no more sugar dissolves, no matter how much you stir. The solution is now saturated.
Factors Affecting Saturation
Several factors significantly impact the saturation point of a solution:
-
Temperature: Temperature plays a crucial role. Generally, the solubility of most solids in liquids increases with increasing temperature. This means you can dissolve more solute in a warmer solvent than in a colder one. However, there are exceptions, such as some gases, whose solubility decreases with increasing temperature.
-
Pressure: Pressure primarily affects the solubility of gases in liquids. Increasing pressure increases the solubility of gases. This is why carbonated drinks are bottled under high pressure: to keep more carbon dioxide dissolved in the solution. The effect of pressure on the solubility of solids is generally negligible.
-
Nature of the Solute and Solvent: The chemical nature of both the solute and the solvent greatly influences solubility. "Like dissolves like" is a common adage in chemistry. Polar solvents (like water) tend to dissolve polar solutes (like sugar), while nonpolar solvents (like oil) dissolve nonpolar solutes (like fats). The intermolecular forces between the solute and solvent molecules determine the extent of solubility. Stronger interactions lead to higher solubility.
-
Presence of Other Substances: The presence of other dissolved substances can significantly alter the solubility of a given solute. This is often referred to as the common ion effect. If you add a substance that contains an ion already present in the solution, the solubility of that original solute will usually decrease. This is because the equilibrium shifts to reduce the concentration of the common ion.
Supersaturated Solutions: Beyond Saturation
It’s possible to create a supersaturated solution, which contains more solute than it can theoretically hold at a given temperature and pressure. These solutions are unstable and are typically prepared by dissolving a large amount of solute in a hot solvent, then carefully cooling the solution without disturbing it. Any small disturbance, like adding a seed crystal or scratching the container, can trigger rapid crystallization, resulting in the precipitation of excess solute. Supersaturated solutions are a fascinating demonstration of metastable states in chemistry.
Practical Applications of Saturated Solutions
The concept of saturation has widespread applications across various fields:
1. Medicine and Pharmaceuticals:
-
Drug Delivery: Understanding saturation is crucial in formulating drug solutions. Saturated solutions provide a consistent and predictable drug concentration.
-
Solubility Testing: Determining the saturation point of a drug is critical for ensuring its bioavailability and effectiveness.
2. Environmental Science:
-
Water Quality: The saturation of dissolved minerals in water bodies is important for maintaining water quality and preventing mineral precipitation that could clog pipes or affect aquatic life.
-
Pollutant Concentration: Determining the saturation level of pollutants in soil or water helps assess environmental risk and guide remediation efforts.
3. Chemical Engineering and Industry:
-
Crystallization: Saturated solutions are frequently used in industrial processes for producing high-purity crystals. Controlled crystallization is used to obtain specific crystal sizes and shapes.
-
Electroplating: Electroplating involves using a saturated solution of the metal ion to ensure a consistent deposition of the metal onto a surface.
4. Food Science:
-
Sugar Solutions: Saturation is crucial in the production of jams, jellies, and candies, where the concentration of sugar determines the texture and consistency of the final product.
-
Salt Solutions: Salt saturation is used in preserving food by controlling microbial growth.
Determining Saturation: Experimental Techniques
Determining whether a solution is saturated, unsaturated, or supersaturated can be done through several experimental methods:
-
Adding More Solute: The simplest method involves adding more solute to the solution. If the solute dissolves, the solution is unsaturated. If the solute does not dissolve, the solution is saturated. If rapid crystallization occurs, the solution was supersaturated.
-
Measuring Solubility: Precise measurement of solubility involves carefully weighing the solute and solvent and measuring the temperature. Different methods exist to ensure accurate measurement, depending on the solute and solvent.
-
Spectroscopic Techniques: Sophisticated spectroscopic techniques can measure the concentration of the solute in a solution and thus determine its saturation status.
Conclusion: The Significance of Saturation
The concept of a saturated solution, representing the maximum solubility of a solute in a solvent, is a fundamental principle in chemistry with far-reaching implications. Understanding the factors that affect saturation and the methods for determining saturation is crucial for various scientific and industrial applications. From pharmaceutical formulations to environmental monitoring and industrial processes, the ability to control and predict saturation plays a vital role in ensuring quality, safety, and efficiency. The exploration of supersaturated solutions further highlights the complexity and fascinating nuances of solution chemistry, opening avenues for continued research and innovation. A comprehensive understanding of saturation is key to unlocking the potential of solutions across diverse fields.
Latest Posts
Latest Posts
-
Where Is Feslimc Magma Plate Voundary
Mar 15, 2025
-
T Test For A Single Sample
Mar 15, 2025
-
Equation For Ionization Of Acetic Acid
Mar 15, 2025
-
Acids And Bases Cannot Mix Together
Mar 15, 2025
-
Linear Programming Do Not Find Minimum Or Maximum
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about A Solution That Contains The Maximum Amount Of Solute . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
