Which State Of Matter Has No Definite Shape Or Volume
Muz Play
Mar 15, 2025 · 6 min read
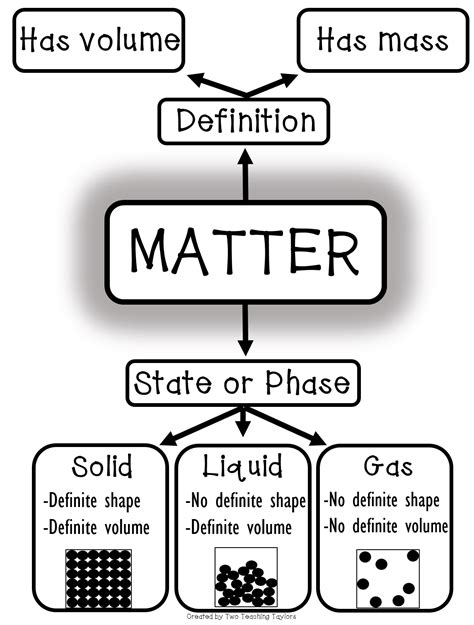
Table of Contents
Which State of Matter Has No Definite Shape or Volume? Understanding Gases
The answer to the question, "Which state of matter has no definite shape or volume?" is unequivocally gas. Unlike solids and liquids, gases exhibit neither a fixed shape nor a fixed volume. This defining characteristic stems from the unique properties of gas particles and their interactions, leading to a fascinating range of behaviors explored in physics and chemistry. This article will delve deep into the properties of gases, contrasting them with solids and liquids, exploring their unique behaviors, and examining their significance in various aspects of our world.
Understanding the Three Fundamental States of Matter
Before focusing specifically on gases, it's crucial to understand the fundamental differences between the three primary states of matter: solid, liquid, and gas. These states are differentiated based on how their constituent particles – atoms, molecules, or ions – are arranged and interact.
Solids: Fixed Shape and Volume
Solids possess a definite shape and volume. Their constituent particles are tightly packed together in a highly ordered arrangement, often forming a crystal lattice structure. The strong intermolecular forces between these particles restrict their movement, leading to rigidity and a fixed shape. Applying force requires significant energy to overcome these strong interactions. Examples include ice, rocks, and metals.
Liquids: Definite Volume, Indefinite Shape
Liquids maintain a definite volume but have an indefinite shape. While their particles are still relatively close together, they have more freedom of movement compared to solids. The intermolecular forces are weaker, allowing particles to slide past each other and adapt to the shape of their container. This fluidity is a key characteristic of liquids. Water, oil, and mercury are common examples.
Gases: Indefinite Shape and Volume
Gases are the chameleons of the matter world. They have neither a definite shape nor a definite volume. Their particles are far apart, with weak intermolecular forces, resulting in almost complete freedom of movement. This allows gases to expand to fill any container they occupy, readily changing shape and volume in response to external factors like pressure and temperature. Air, helium, and carbon dioxide are familiar examples.
The Unique Properties of Gases: A Deeper Dive
The lack of definite shape and volume in gases is a direct consequence of several key properties:
1. Compressibility
Gases are highly compressible. The large spaces between gas particles allow them to be squeezed closer together, reducing the volume significantly with increased pressure. This property is exploited in various applications, such as compressed air cylinders used for tools and scuba diving.
2. Expansibility
Gases are readily expandable. If the pressure is reduced or the temperature is increased, the gas particles move further apart, resulting in an increase in volume. This explains why balloons inflate when filled with air or helium.
3. Diffusivity
Gases exhibit high diffusivity. Due to the constant random motion of gas particles and the significant inter-particle distances, they readily mix with other gases. This explains why odors spread quickly through the air.
4. Low Density
Compared to solids and liquids, gases have very low densities. The large spaces between gas particles mean that a given volume of gas contains far fewer particles than the same volume of a solid or liquid. This low density is why gases are often buoyant.
Kinetic Molecular Theory: Explaining Gas Behavior
The kinetic molecular theory (KMT) provides a microscopic explanation for the macroscopic properties of gases. KMT postulates that:
- Gas particles are in constant, random motion.
- The volume of individual gas particles is negligible compared to the total volume of the gas.
- There are no significant attractive or repulsive forces between gas particles.
- Collisions between gas particles are perfectly elastic (no energy loss).
- The average kinetic energy of gas particles is directly proportional to the absolute temperature.
These postulates help explain the observed behavior of gases, including their compressibility, expansibility, and diffusivity. The random motion of particles leads to their spreading out to fill available space, explaining the indefinite shape and volume. The negligible volume of particles and the absence of strong intermolecular forces allow for compressibility and expansibility.
Factors Affecting Gas Behavior: Pressure, Temperature, and Volume
Three primary factors influence the behavior of gases: pressure, temperature, and volume. These are related through gas laws, which provide mathematical relationships describing their interactions.
1. Pressure (P)
Pressure is the force exerted by gas particles per unit area. Higher gas particle density leads to higher pressure, as more frequent collisions occur with the container walls. Pressure is typically measured in atmospheres (atm), pascals (Pa), or millimeters of mercury (mmHg).
2. Volume (V)
Volume is the amount of space occupied by a gas. It's directly proportional to the number of gas particles and inversely proportional to the pressure. Volume is usually measured in liters (L) or cubic meters (m³).
3. Temperature (T)
Temperature reflects the average kinetic energy of gas particles. Higher temperatures lead to faster particle motion, resulting in increased pressure and volume if the container is flexible. Temperature is measured in Kelvin (K).
Ideal Gas Law: A Unified Description
The ideal gas law combines the relationships between pressure, volume, temperature, and the number of moles (n) of gas into a single equation: PV = nRT, where R is the ideal gas constant. This law provides a useful approximation for the behavior of many gases under moderate conditions, although it has limitations for gases at high pressures or low temperatures.
Real Gases vs. Ideal Gases: Deviations from Ideality
The ideal gas law is a simplification. Real gases exhibit deviations from ideality, particularly at high pressures and low temperatures. At high pressures, the volume of the gas particles becomes significant relative to the total volume, and intermolecular forces become more prominent. These factors lead to deviations from the predictions of the ideal gas law. Different equations of state, such as the van der Waals equation, attempt to better model the behavior of real gases.
Applications of Gas Properties: A Wide-Ranging Impact
The unique properties of gases have profound implications in various fields:
1. Atmospheric Science: Weather Patterns and Climate Change
Gases in the atmosphere, such as nitrogen, oxygen, and carbon dioxide, play vital roles in weather patterns and climate regulation. Understanding their behavior is critical for predicting weather phenomena and addressing climate change.
2. Industrial Processes: Chemical Reactions and Manufacturing
Many industrial processes involve gases, including chemical reactions, manufacturing, and energy production. Controlling gas properties is crucial for optimizing these processes.
3. Medical Applications: Anesthesia and Respiratory Therapy
Gases like oxygen, nitrous oxide, and anesthetic agents have crucial applications in medicine, supporting respiration and anesthesia.
4. Everyday Life: Respiration and Combustion
Gases are essential for respiration and combustion, processes fundamental to life and energy production.
Conclusion: The Ever-Present and Essential Nature of Gases
In conclusion, gases are a fundamental state of matter distinguished by their indefinite shape and volume. This characteristic arises from the weak intermolecular forces and high degree of particle freedom. Their behavior is governed by fundamental laws such as the ideal gas law, though deviations from ideality occur under extreme conditions. The properties and behaviors of gases have wide-ranging implications across diverse fields, impacting weather patterns, industrial processes, medical applications, and countless aspects of our daily lives. Understanding the unique properties of gases is crucial for comprehending and harnessing their importance in the natural world and various technological applications.
Latest Posts
Latest Posts
-
Do Valence Electrons Have The Most Energy
Mar 17, 2025
-
Element Vs Compound Vs Homogeneous Vs Heterogeneous
Mar 17, 2025
-
For An Exothermic Reaction The Products
Mar 17, 2025
-
Fallacies Divide Into Roughly Two Kinds
Mar 17, 2025
-
An Organism That Cannot Grow Without Oxygen Is A An
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Has No Definite Shape Or Volume . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
