How Do Buffers Resist Changes In Ph
Muz Play
Mar 16, 2025 · 6 min read
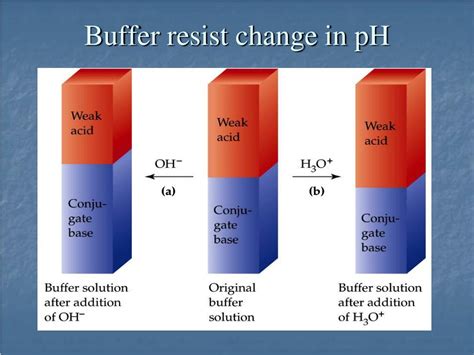
Table of Contents
How Do Buffers Resist Changes in pH?
Buffers are solutions that resist changes in pH upon the addition of small amounts of acid or base. This crucial property is fundamental to many biological and chemical processes, maintaining a stable environment necessary for optimal function. Understanding how buffers achieve this resistance is key to appreciating their significance. This article will delve deep into the mechanisms behind buffer action, exploring various types of buffers and their applications.
The Chemistry of Buffer Solutions
At the heart of a buffer's ability to resist pH changes lies the equilibrium between a weak acid and its conjugate base (or a weak base and its conjugate acid). This equilibrium is described by the Henderson-Hasselbalch equation:
pH = pKa + log([A⁻]/[HA])
Where:
- pH is the solution's pH.
- pKa is the negative logarithm of the acid dissociation constant (Ka) of the weak acid. The pKa represents the pH at which the weak acid is half-dissociated.
- [A⁻] is the concentration of the conjugate base.
- [HA] is the concentration of the weak acid.
This equation illustrates the dynamic interplay between the acid and its conjugate base. When a small amount of strong acid is added, the conjugate base (A⁻) reacts with the added H⁺ ions, forming more of the weak acid (HA). This reaction minimizes the increase in H⁺ concentration and therefore minimizes the change in pH. Conversely, when a small amount of strong base is added, the weak acid (HA) reacts with the added OH⁻ ions, forming more of the conjugate base (A⁻) and water. This neutralizes the added OH⁻ and prevents a significant pH increase.
The effectiveness of a buffer depends on several factors:
-
The pKa of the weak acid: A buffer is most effective when the pH of the solution is close to the pKa of the weak acid. This is because at this pH, the concentrations of the weak acid and its conjugate base are roughly equal, allowing for maximum buffering capacity.
-
The concentrations of the weak acid and its conjugate base: A higher concentration of both the weak acid and its conjugate base leads to a greater buffering capacity. A more concentrated buffer can absorb more added acid or base before significant pH changes occur.
-
The amount of strong acid or base added: Buffers are effective only within a limited range. Adding a large amount of strong acid or base will eventually overwhelm the buffer's capacity, leading to a significant pH change.
Types of Buffer Solutions
Various types of buffer solutions exist, each with its own characteristics and applications. Some common examples include:
1. Acetate Buffer:
This buffer is composed of acetic acid (CH₃COOH) and its conjugate base, sodium acetate (CH₃COONa). Its pKa is approximately 4.76, making it suitable for buffering solutions in the pH range of 3.76 to 5.76. Acetate buffers are commonly used in biochemical experiments and in the pharmaceutical industry.
2. Phosphate Buffer:
Phosphate buffers are frequently used in biological systems due to the prevalence of phosphate groups in biological molecules. They typically consist of a mixture of monobasic sodium phosphate (NaH₂PO₄) and dibasic sodium phosphate (Na₂HPO₄). Phosphate buffers have different pKa values depending on the specific phosphate species involved, allowing for buffering over a range of pH values.
3. Tris Buffer:
Tris (tris(hydroxymethyl)aminomethane) is a widely used buffer in biochemistry and molecular biology. It's a weak base, and its buffering capacity is often adjusted by adding a strong acid like HCl to lower the pH. Tris buffers are particularly useful for maintaining a stable pH in biological experiments because they have minimal interference with biological processes.
4. Carbonate Buffer:
The carbonate buffer system is crucial for maintaining the pH of blood. It involves carbonic acid (H₂CO₃) and its conjugate base, bicarbonate (HCO₃⁻). This system helps regulate blood pH by reacting with added acids or bases, preventing significant fluctuations.
5. HEPES Buffer:
HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) is another popular buffer in biological research. It's a zwitterionic buffer, meaning it carries both positive and negative charges, providing excellent buffering capacity over a wide pH range, often used in cell culture.
The Importance of Buffers in Biological Systems
Buffers play a vital role in maintaining the pH of biological systems. Slight changes in pH can drastically affect the activity of enzymes, the structure of proteins, and the function of other biological molecules. Several examples highlight the significance of buffers in living organisms:
-
Blood pH regulation: The carbonate buffer system is crucial for maintaining the pH of blood within a narrow range (7.35-7.45). Deviations from this range can lead to acidosis or alkalosis, which can be life-threatening.
-
Enzyme activity: Many enzymes have optimal pH ranges within which they function effectively. Buffers help maintain these optimal pH conditions, ensuring proper enzyme activity.
-
Protein structure: The three-dimensional structure of proteins is highly sensitive to pH changes. Buffers help stabilize the protein structure by preventing significant pH fluctuations.
-
Cell culture: In cell culture, buffers are essential for maintaining a stable pH, ensuring the cells grow and function optimally.
Applications of Buffers Beyond Biology
The applications of buffers extend beyond biological systems into various chemical and industrial processes:
-
Analytical chemistry: Buffers are frequently used in analytical techniques to control the pH of solutions during titrations and other analytical procedures.
-
Electrochemistry: Buffers are necessary for maintaining a stable pH in electrochemical cells and electrodes, ensuring accurate and reliable measurements.
-
Pharmaceutical industry: Buffers are used in the formulation of many pharmaceuticals to ensure stability and effectiveness.
-
Food industry: Buffers are used in food preservation to control the pH and prevent spoilage.
-
Photography: Certain photographic processes rely on specific pH ranges, maintained using buffers.
Choosing the Right Buffer
Selecting the appropriate buffer for a specific application requires careful consideration of several factors:
-
Desired pH range: The pKa of the buffer should be close to the desired pH.
-
Buffering capacity: The concentration of the buffer should be sufficient to resist anticipated pH changes.
-
Ionic strength: The ionic strength of the buffer can influence the activity of ions and biological molecules.
-
Solubility: The buffer components should be soluble in the solvent used.
-
Potential interactions: The buffer should not interfere with the specific application, particularly in biological systems where the buffer may interact with biological molecules.
Conclusion
Buffers are indispensable tools in chemistry, biochemistry, and various industrial applications. Their ability to resist pH changes stems from the equilibrium between a weak acid and its conjugate base (or weak base and conjugate acid). Understanding the principles behind buffer action, including the Henderson-Hasselbalch equation, is crucial for selecting and utilizing buffers effectively. The diverse types of buffers available, each with its unique properties, provide solutions for a wide range of pH control needs. The careful choice of buffer is vital for maintaining stable conditions and optimal performance in countless applications. From maintaining the pH of our blood to ensuring the proper function of enzymes in biological systems and enabling precise chemical reactions, buffers are essential components of many crucial processes.
Latest Posts
Latest Posts
-
Electric Potential From A Point Charge
Mar 17, 2025
-
Whats The Derivative Of A Constant
Mar 17, 2025
-
Differential Rate Law For Zero Order Reaction
Mar 17, 2025
-
Cell The Basic Unit Of Life
Mar 17, 2025
-
An Increase In The Aggregate Expenditures Schedule
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about How Do Buffers Resist Changes In Ph . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
