How Do You Calculate Temperature Change
Muz Play
Mar 23, 2025 · 5 min read
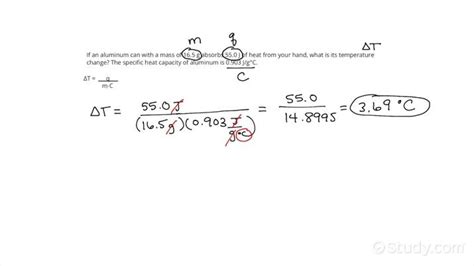
Table of Contents
How Do You Calculate Temperature Change? A Comprehensive Guide
Calculating temperature change might seem straightforward, but understanding the nuances is crucial for accuracy and applying the knowledge to various scientific and everyday situations. This comprehensive guide delves into the different methods, formulas, and considerations involved in accurately calculating temperature changes, catering to both beginners and those seeking a deeper understanding.
Understanding the Basics: Temperature Scales and Units
Before diving into calculations, it's essential to grasp the fundamental concepts of temperature scales and their units. The most commonly used scales are:
- Celsius (°C): Based on the freezing (0°C) and boiling (100°C) points of water at standard atmospheric pressure.
- Fahrenheit (°F): Another widely used scale, where water freezes at 32°F and boils at 212°F.
- Kelvin (K): The absolute temperature scale, where 0 K represents absolute zero—the theoretical absence of all thermal energy. There are no negative Kelvin temperatures.
Knowing these scales is crucial because conversion between them is often necessary before performing calculations.
Converting Between Temperature Scales
The formulas for converting between Celsius, Fahrenheit, and Kelvin are:
- Celsius to Fahrenheit: °F = (°C × 9/5) + 32
- Fahrenheit to Celsius: °C = (°F - 32) × 5/9
- Celsius to Kelvin: K = °C + 273.15
- Kelvin to Celsius: °C = K - 273.15
- Fahrenheit to Kelvin: K = (°F + 459.67) × 5/9
- Kelvin to Fahrenheit: °F = (K × 9/5) - 459.67
Example: Converting 25°C to Fahrenheit:
°F = (25 × 9/5) + 32 = 77°F
Calculating Simple Temperature Changes
The simplest form of temperature change calculation involves subtracting the initial temperature from the final temperature.
Formula: ΔT = T<sub>final</sub> - T<sub>initial</sub>
Where:
- ΔT represents the change in temperature.
- T<sub>final</sub> is the final temperature.
- T<sub>initial</sub> is the initial temperature.
Important Note: Ensure both temperatures are in the same units before performing the subtraction.
Example: If the initial temperature of a substance is 10°C and the final temperature is 25°C, the temperature change is:
ΔT = 25°C - 10°C = 15°C
Calculating Temperature Change with Heat Transfer
In many scenarios, temperature change is a direct result of heat transfer. The relationship between heat transfer (Q), mass (m), specific heat capacity (c), and temperature change (ΔT) is described by the following equation:
Formula: Q = mcΔT
Where:
- Q: Heat transferred (measured in Joules, J)
- m: Mass of the substance (measured in kilograms, kg)
- c: Specific heat capacity of the substance (measured in Joules per kilogram per Kelvin, J/kg·K or J/kg·°C. The change in Celsius and Kelvin are equivalent)
- ΔT: Change in temperature (measured in Kelvin or Celsius)
This formula allows us to calculate the temperature change given the heat transfer, mass, and specific heat capacity, or vice-versa. We can rearrange the formula to solve for any of these variables:
- To find ΔT: ΔT = Q / (mc)
- To find Q: Q = mcΔT
- To find m: m = Q / (cΔT)
- To find c: c = Q / (mΔT)
Example: If 5000 J of heat is added to 2 kg of water (specific heat capacity of water is approximately 4186 J/kg·°C), what is the temperature change?
ΔT = Q / (mc) = 5000 J / (2 kg × 4186 J/kg·°C) ≈ 0.6°C
Factors Affecting Temperature Change
Several factors can influence the accuracy of temperature change calculations:
1. Specific Heat Capacity:
Different substances have different specific heat capacities. Water, for instance, has a relatively high specific heat capacity, meaning it requires a significant amount of heat to raise its temperature. Metals, on the other hand, generally have lower specific heat capacities.
2. Heat Loss/Gain to the Surroundings:
In real-world scenarios, heat can be lost or gained to the surrounding environment. This can significantly affect the actual temperature change, leading to discrepancies between calculated and observed values. Insulation helps minimize these effects.
3. Phase Changes:
The formulas above assume that the substance remains in the same phase (solid, liquid, or gas). During phase transitions (melting, boiling, etc.), heat is absorbed or released without a corresponding temperature change. Calculating temperature changes during phase transitions requires different equations and considerations, involving latent heat.
4. Pressure:
Pressure can also influence temperature, particularly in gases. Changes in pressure can cause temperature changes even without heat transfer, described by principles of thermodynamics.
5. Accuracy of Measurement Instruments:
The accuracy of the calculated temperature change is directly dependent on the accuracy of the temperature measurements. Using calibrated and reliable thermometers is essential for obtaining accurate results.
Advanced Calculations: Using Calorimetry
Calorimetry is a technique used to experimentally determine the heat transferred during a process, often involving a calorimeter – a device designed to minimize heat exchange with the surroundings. By measuring the temperature change of the calorimeter and its contents, the heat transferred can be calculated, and then used to determine other variables like specific heat capacity or the heat of reaction.
Applications of Temperature Change Calculations
The principles of calculating temperature change are applied across various fields:
- Chemistry: Determining the heat of reaction, specific heat capacities, and enthalpy changes.
- Physics: Understanding heat transfer, thermodynamics, and thermal properties of materials.
- Engineering: Designing efficient heating and cooling systems, thermal management of electronic devices, and material selection.
- Meteorology: Forecasting weather patterns and analyzing climate change.
- Food Science: Controlling cooking processes and food preservation.
- Medicine: Monitoring body temperature and managing thermal therapies.
Conclusion
Accurately calculating temperature change is fundamental to numerous scientific and engineering disciplines. While the basic formula is straightforward, understanding the underlying principles, considering factors like specific heat capacity, heat loss, and phase transitions, and using appropriate techniques like calorimetry are crucial for obtaining accurate and reliable results. The ability to perform these calculations accurately provides a powerful tool for analyzing and understanding thermal processes in a wide array of contexts. Remember to always ensure your units are consistent and to choose the appropriate formula based on the specific problem you're addressing.
Latest Posts
Latest Posts
-
What Does The Top Command Do In Linux
Mar 25, 2025
-
Picture Of A Cell In Interphase
Mar 25, 2025
-
In Biology How Is A Weed Defined
Mar 25, 2025
-
What Is The Ph Of The Neutral Solution
Mar 25, 2025
-
Electric Field Of A Uniformly Charged Disk
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Do You Calculate Temperature Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
