What Is The Ph Of The Neutral Solution
Muz Play
Mar 25, 2025 · 6 min read
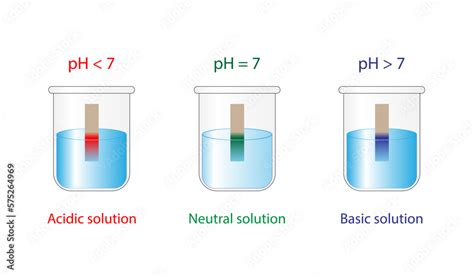
Table of Contents
What is the pH of a Neutral Solution? A Deep Dive into pH, Acidity, and Alkalinity
The seemingly simple question, "What is the pH of a neutral solution?" opens a door to a fascinating world of chemistry, impacting everything from our bodily functions to industrial processes. Understanding pH is crucial for various fields, from agriculture and medicine to environmental science and manufacturing. This comprehensive guide will explore the concept of pH, explain what constitutes a neutral solution, delve into the pH scale, and discuss the implications of pH variations in different contexts.
Understanding pH: A Measure of Hydrogen Ion Concentration
pH, a term derived from the "potential of hydrogen," is a logarithmic scale used to specify the acidity or basicity (alkalinity) of an aqueous solution. It measures the concentration of hydrogen ions (H⁺) present in a solution. The more hydrogen ions present, the more acidic the solution is, and conversely, the fewer hydrogen ions, the more alkaline (basic) it becomes.
The scale ranges from 0 to 14, with:
- pH 7: Representing a neutral solution. This means the concentration of hydrogen ions (H⁺) is equal to the concentration of hydroxide ions (OH⁻).
- pH < 7: Indicating an acidic solution. The concentration of hydrogen ions is higher than the concentration of hydroxide ions. The lower the pH value, the stronger the acid.
- pH > 7: Indicating an alkaline (basic) solution. The concentration of hydroxide ions is higher than the concentration of hydrogen ions. The higher the pH value, the stronger the base.
It's crucial to understand that the pH scale is logarithmic. This means that a change of one pH unit represents a tenfold change in the concentration of hydrogen ions. For instance, a solution with a pH of 4 is ten times more acidic than a solution with a pH of 5, and one hundred times more acidic than a solution with a pH of 6.
The Role of Water in pH
Water (H₂O) plays a vital role in determining pH. Pure water undergoes a process called self-ionization, where a small fraction of water molecules dissociate into hydrogen ions (H⁺) and hydroxide ions (OH⁻):
H₂O ⇌ H⁺ + OH⁻
This equilibrium reaction maintains a constant concentration of both ions at 25°C. This concentration is 1 x 10⁻⁷ moles per liter for both H⁺ and OH⁻. The product of these concentrations, known as the ion product of water (Kw), is always 1 x 10⁻¹⁴ at 25°C. This constant relationship is fundamental to understanding pH.
What Makes a Solution Neutral?
A solution is considered neutral when the concentration of hydrogen ions (H⁺) is equal to the concentration of hydroxide ions (OH⁻). This equilibrium is precisely what is observed in pure water at 25°C, resulting in a pH of 7. It's important to note that the neutrality of a solution depends on temperature. At temperatures other than 25°C, the Kw changes, affecting the pH of a neutral solution. At higher temperatures, Kw increases, meaning the pH of neutral water becomes slightly less than 7. At lower temperatures, the opposite happens.
Factors Affecting pH
Numerous factors can influence the pH of a solution, including:
- Temperature: As mentioned, temperature significantly impacts the self-ionization of water and thus the pH of a neutral solution.
- Dissolved Substances: The presence of acids, bases, or salts in the solution will alter the concentration of hydrogen and hydroxide ions, shifting the pH away from neutrality. Strong acids and bases have a more pronounced effect than weak ones.
- Concentration: The concentration of an acid or base directly affects its impact on pH. A more concentrated solution will exhibit a greater change in pH.
- Buffer Solutions: Buffer solutions are designed to resist changes in pH upon the addition of small amounts of acid or base. They are crucial in maintaining stable pH levels in biological systems and chemical processes.
Measuring pH: Methods and Tools
Precise pH measurement is essential across various scientific and industrial applications. Several methods are used to determine the pH of a solution:
- pH Meter: This electronic instrument is the most common and accurate method for pH measurement. A pH meter utilizes a special electrode (pH electrode or glass electrode) that responds to the concentration of hydrogen ions in a solution. The voltage generated by the electrode is then converted to a pH reading.
- pH Indicators: These are chemical substances that change color depending on the pH of the solution. Common examples include litmus paper, bromothymol blue, and phenolphthalein. While less precise than a pH meter, pH indicators provide a quick visual estimate of pH.
- Titration: This is a laboratory technique that involves gradually adding a solution of known concentration (titrant) to a solution of unknown concentration until a specific reaction is complete. Acid-base titrations are used to determine the concentration of an unknown acid or base, which can then be used to calculate the pH.
The Significance of pH in Different Fields
The pH level is a critical parameter in various applications:
1. Biology and Medicine:
- Human Body: The pH of different bodily fluids is strictly regulated within specific ranges. Blood, for instance, maintains a slightly alkaline pH (around 7.4), crucial for proper enzyme function and overall health. Deviations from this range can lead to severe health issues like acidosis or alkalosis.
- Digestive System: The pH of the stomach is highly acidic (around 1.5-3.5), which is necessary for the digestion of food and the killing of harmful bacteria.
- Drug Delivery: The pH of a drug solution can influence its absorption and effectiveness in the body.
2. Agriculture:
- Soil pH: The pH of soil dramatically affects plant growth. Different plants thrive in different pH ranges. Maintaining the optimal pH is crucial for healthy plant development and nutrient uptake.
- Irrigation Water: The pH of irrigation water can affect soil pH and plant health.
3. Environmental Science:
- Water Quality: Monitoring the pH of water bodies is essential for assessing water quality and identifying potential pollution. Acid rain, for example, lowers the pH of lakes and rivers, potentially harming aquatic life.
- Pollution Control: pH adjustments are frequently employed in wastewater treatment processes to optimize the removal of pollutants.
4. Industry:
- Chemical Processes: Many chemical reactions are pH-sensitive, and controlling the pH is essential for achieving desired outcomes and product quality.
- Food and Beverage Industry: pH control is vital in food preservation, fermentation processes (e.g., winemaking, cheese production), and maintaining food quality.
Conclusion: The Importance of Understanding pH
The pH of a neutral solution, while seemingly simple, serves as a foundational concept in understanding acidity, alkalinity, and their widespread impact across diverse fields. The precise measurement and control of pH are critical for maintaining health, preserving the environment, and optimizing various industrial processes. From the delicate balance of human blood pH to the complexities of soil chemistry, the significance of pH extends far beyond a simple numerical value. It's a fundamental principle that underpins many essential processes in our world, highlighting the importance of understanding this key concept in chemistry.
Latest Posts
Latest Posts
-
Is Mrna Processing Is Same For Prokaryote And Eukaryote
Mar 26, 2025
-
Magnetic Field For A Bar Magnet
Mar 26, 2025
-
What Is A Common Property Of Metals
Mar 26, 2025
-
Curriculum Models In Early Childhood Education
Mar 26, 2025
-
Using Hesss Law To Calculate Net Reaction Enthalpy
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Ph Of The Neutral Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
