What Is A Common Property Of Metals
Muz Play
Mar 26, 2025 · 7 min read
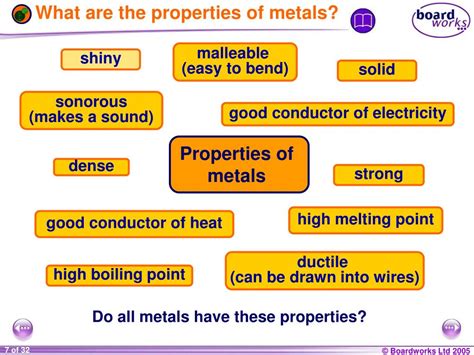
Table of Contents
What is a Common Property of Metals? Delving into the Characteristics that Define Metals
Metals are ubiquitous in our daily lives, from the smartphones we use to the buildings we inhabit. This widespread presence stems from their unique and valuable properties, making them indispensable in countless applications. But what exactly defines a metal? This article explores the common properties of metals, diving deep into their physical and chemical characteristics, and examining exceptions to these rules. We'll also touch upon why these properties make metals so crucial to our technological advancements and everyday lives.
Key Physical Properties of Metals
Several physical characteristics readily distinguish metals from other materials. These properties are largely driven by the unique structure and bonding of metal atoms.
1. Luster: The Shiny Appeal of Metals
One of the most immediately noticeable properties of metals is their luster, also known as their shine or metallic sheen. This characteristic arises from the way metal atoms interact with light. The electrons in the outermost shell of metal atoms are loosely bound and are free to move throughout the metal structure. These delocalized electrons readily absorb and re-emit light across the visible spectrum, creating the characteristic metallic sheen. This is why metals often appear shiny and reflective. However, the degree of luster can vary depending on the specific metal and its surface condition. For instance, a highly polished silver surface will exhibit a greater luster than a tarnished one.
2. Malleability and Ductility: Shaping Metals
Metals are renowned for their malleability and ductility. Malleability refers to the ability of a metal to be deformed under compressive stress; it can be hammered or rolled into sheets without breaking. Ductility, on the other hand, refers to the ability of a metal to be drawn into wires. Both these properties are a direct result of the "sea" of delocalized electrons in the metallic structure. These electrons act as a buffer, allowing the metal atoms to slide past each other without disrupting the metallic bond. This contrasts sharply with ionic or covalent solids, where strong directional bonds would cause the material to fracture under similar stress.
Examples of highly malleable and ductile metals include gold, silver, and copper, which are frequently used in jewelry making and electrical wiring respectively. Iron, while less ductile than gold, is still malleable enough for forging and shaping.
3. Conductivity: Efficient Electron Flow
Metals are excellent conductors of both electricity and heat. This superior conductivity is intrinsically linked to the delocalized electrons. These mobile electrons can readily transport both electrical charge and thermal energy throughout the metal structure. The ease with which electrons move accounts for the high electrical conductivity of metals, which is crucial in electrical wiring and various electronic components. Similarly, the free movement of electrons facilitates efficient heat transfer, making metals ideal for applications like cookware and heat sinks. Silver is the best electrical conductor, followed closely by copper, which is more widely used due to its lower cost.
4. Density: A Measure of Compactness
Metals generally possess relatively high density, meaning that a given volume of metal will have a large mass compared to other materials. This high density is a result of the close packing of atoms in the metallic lattice structure. The strength of metallic bonds results in atoms being tightly packed together, leading to a higher density. While exceptions exist (e.g., lithium is a relatively low-density metal), the majority of metals are denser than non-metals. This density is an important consideration in engineering applications where weight is a factor.
5. Hardness and Strength: Resistance to Deformation
Many metals exhibit high hardness and strength, resisting deformation under stress. However, this is not a universal property; some metals are softer than others. The hardness and strength of a metal are influenced by several factors, including the type of metal, its purity, and its crystal structure. The strong metallic bonds contribute to the overall strength of the metal lattice. Alloying, a process of mixing different metals, is often used to enhance the strength and hardness of metals, creating materials like steel (an alloy of iron and carbon) which are significantly stronger than pure iron.
6. Melting and Boiling Points: Temperature Resistance
Metals generally have relatively high melting and boiling points, meaning they require significant energy input to change their state from solid to liquid or liquid to gas. This is again due to the strong metallic bonds holding the atoms together. The stronger the bonds, the more energy needed to break them. However, there's significant variation amongst metals; Mercury, for example, is a liquid at room temperature, while Tungsten possesses an exceptionally high melting point.
Key Chemical Properties of Metals
Besides their physical attributes, metals display several defining chemical characteristics.
1. Reactivity: Interaction with Other Elements
The reactivity of metals varies greatly depending on their position in the periodic table. Metals are generally characterized by their tendency to lose electrons and form positive ions (cations). This is because the outermost electrons are relatively loosely bound. Highly reactive metals, such as alkali metals (Group 1), readily lose electrons and react vigorously with water and air. Less reactive metals, such as gold and platinum, are much more resistant to corrosion. The reactivity of a metal is crucial in determining its applications and how it needs to be handled.
2. Corrosion: Degradation Over Time
Many metals are susceptible to corrosion, a process where the metal reacts with its environment, leading to its gradual degradation. This is often a redox reaction involving the metal losing electrons and reacting with substances like oxygen or water. Rusting of iron is a classic example of corrosion. The formation of a protective oxide layer on certain metals (like aluminum) can act as a barrier against further corrosion, preventing significant degradation. Understanding corrosion is essential in selecting appropriate materials for different applications and employing protective measures.
3. Oxidation: Losing Electrons
Metals tend to undergo oxidation, a chemical process involving the loss of electrons. This often leads to the formation of metal oxides or other compounds. The ease with which a metal undergoes oxidation is directly related to its reactivity. Highly reactive metals readily oxidize, while less reactive metals are more resistant. Oxidation plays a significant role in various chemical processes, from the formation of rust to the functioning of batteries.
4. Formation of Alloys: Mixing Metals
Metals readily form alloys with other metals or non-metals. An alloy is a mixture of two or more elements, where at least one element is a metal. Alloying significantly alters the properties of the constituent metals, leading to materials with enhanced strength, hardness, corrosion resistance, or other desirable characteristics. Steel, brass, and bronze are well-known examples of alloys with improved properties compared to their constituent metals. The process of alloying is essential in materials science for tailoring the properties of metals to specific applications.
Exceptions and Nuances: Not All Metals are Created Equal
While the properties discussed above are generally characteristic of metals, it's crucial to acknowledge that there are exceptions and nuances. Not all metals exhibit these properties to the same degree. For example:
- Mercury: Is a liquid at room temperature, unlike most metals.
- Bismuth: Is a brittle metal, lacking the malleability and ductility typical of other metals.
- Some transition metals: Exhibit a range of oxidation states, leading to varied reactivity and chemical behavior.
- Alloys: The properties of alloys can significantly deviate from the properties of the constituent metals.
Conclusion: The Importance of Metallic Properties
The common properties of metals—their luster, malleability, ductility, conductivity, density, hardness, high melting points, reactivity, and tendency to form alloys—are interconnected and arise from the fundamental nature of metallic bonding. These properties render metals indispensable across diverse fields, from construction and transportation to electronics and medicine. Understanding these properties is crucial for engineers, scientists, and anyone seeking to utilize the unique capabilities of these essential materials. Further research into the intricacies of metallic behavior continues to drive innovation and improve our understanding of these fundamental materials of our world. The continuous exploration of these properties ensures that metals will continue to play a pivotal role in shaping future technologies and our world.
Latest Posts
Latest Posts
-
Label The Structures Of The Upper Respiratory System
Mar 29, 2025
-
Assigning R And S Configuration Practice
Mar 29, 2025
-
Does Higher Pka Mean Stronger Acid
Mar 29, 2025
-
In A Chemical Reaction What Are The Reactants And Products
Mar 29, 2025
-
What Elements Are Gases At Room Temperature
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is A Common Property Of Metals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
