What Elements Are Gases At Room Temperature
Muz Play
Mar 29, 2025 · 5 min read
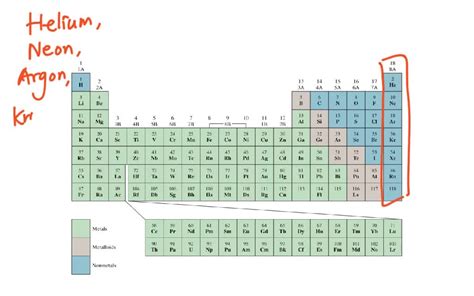
Table of Contents
What Elements Are Gases at Room Temperature?
The world around us is teeming with matter in its various states – solid, liquid, and gas. While solids and liquids are relatively easy to visualize, the gaseous state often requires a deeper understanding of atomic structure and intermolecular forces. This article delves into the fascinating world of elements that exist as gases at room temperature (typically defined as 20-25°C or 68-77°F), exploring their properties, uses, and significance.
The Noble Gases: A Family of Inert Gases
The most well-known group of elements that are gases at room temperature are the noble gases. These include helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and the radioactive radon (Rn). Their "noble" nature stems from their exceptionally stable electron configurations. They have completely filled outer electron shells, meaning they rarely participate in chemical reactions, hence their inertness.
Helium (He): The Lighter-Than-Air Wonder
Helium, the second lightest element, is incredibly versatile. Its low density makes it ideal for filling balloons and blimps, giving them buoyancy. In scientific research, helium is used as a coolant in cryogenics, enabling the study of materials at extremely low temperatures. Its inert nature also makes it suitable for use in arc welding and as a protective atmosphere in various industrial processes. Helium's unique properties stem directly from its small atomic size and weak intermolecular forces. This allows it to remain a gas even at very low temperatures.
Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn): Diverse Applications
Neon, argon, krypton, xenon, and radon, while less common than helium, find niche applications. Neon's characteristic red glow is famously used in neon signs. Argon, being abundant and inert, is used as a shielding gas in welding and in incandescent light bulbs to prevent filament oxidation. Krypton, xenon, and radon have more specialized applications, particularly in lighting and medical imaging. The heavier noble gases exhibit slightly stronger intermolecular forces compared to helium, but these forces remain too weak to cause liquefaction at room temperature.
Diatomic Gases: Bonding for Stability
Several elements exist as diatomic molecules – two atoms of the same element bonded together – at room temperature. These include hydrogen (H₂), nitrogen (N₂), oxygen (O₂), fluorine (F₂), and chlorine (Cl₂). The diatomic nature arises from the elements' desire to achieve a stable electron configuration, often by sharing electrons in a covalent bond.
Hydrogen (H₂): A Promising Fuel Source
Hydrogen is the most abundant element in the universe but is relatively scarce in its pure form on Earth. It's a highly reactive gas, readily forming compounds with other elements. However, its clean combustion properties, producing only water as a byproduct, make it a promising fuel source for a sustainable future. Hydrogen's low boiling point is a result of weak van der Waals forces between its diatomic molecules.
Nitrogen (N₂): Essential for Life
Nitrogen is an essential component of all living organisms, forming part of amino acids, proteins, and nucleic acids (DNA and RNA). Most nitrogen in the atmosphere exists as diatomic nitrogen (N₂), which is relatively inert due to the strong triple bond between the two nitrogen atoms. This inertness is crucial for the survival of life, as reactive nitrogen species can be damaging. The triple bond in N₂ creates a very stable molecule, requiring significant energy to break the bond. This high bond energy contributes to nitrogen remaining a gas at room temperature.
Oxygen (O₂): Crucial for Respiration
Oxygen is vital for respiration in most living organisms. It's a highly reactive gas, readily participating in combustion and oxidation reactions. The presence of oxygen in the atmosphere is a key factor in the Earth's habitability. The double bond in O₂ provides stability, but not as much as the triple bond in N₂. This leads to a slightly higher reactivity compared to nitrogen.
Fluorine (F₂) and Chlorine (Cl₂): Reactive Halogens
Fluorine and chlorine are halogens, a group of highly reactive nonmetals. Fluorine is the most reactive element, readily forming compounds with almost all other elements. Chlorine, while less reactive than fluorine, is still a potent oxidizing agent. Both fluorine and chlorine exist as diatomic molecules due to their tendency to gain an electron and achieve a stable octet. Their relatively stronger intermolecular forces compared to hydrogen or noble gases influence their properties.
Other Gaseous Elements at Room Temperature
Beyond noble gases and diatomic molecules, a few other elements exist as gases at room temperature under standard conditions. These elements often showcase unique properties and applications.
Bromine (Br₂): The Only Liquid Nonmetal
While most nonmetals are solids or gases at room temperature, bromine is an exception. Although it exists as a liquid under standard conditions, its high vapor pressure at room temperature means it readily evaporates, forming a reddish-brown gas with a pungent, irritating odor. Bromine's unique liquid state at room temperature is a consequence of relatively stronger intermolecular forces (van der Waals forces) than those found in gases but still weaker than those in solids.
It's crucial to note that the state of matter is dependent on temperature and pressure. While these elements are gases at room temperature and standard atmospheric pressure, changing these conditions can result in a phase transition (e.g., liquefaction or solidification). Furthermore, some elements might exist as gases at higher temperatures or under reduced pressures, while other elements, normally solid or liquid at room temperature, may become gaseous when heated sufficiently.
Conclusion: A Dynamic World of Gases
The elements that exist as gases at room temperature represent a diverse range of chemical properties and applications. From the inert noble gases to the highly reactive halogens, these elements play essential roles in various scientific, industrial, and biological processes. Understanding their atomic structure, bonding, and intermolecular forces is crucial for appreciating their unique characteristics and the significant impact they have on the world around us. Further exploration of these elements can lead to exciting discoveries and innovative applications in the future. The study of these gaseous elements highlights the dynamic nature of matter and the interplay between atomic structure and macroscopic properties. Each element presents unique characteristics and applications, making their study a fascinating and ongoing pursuit in the field of chemistry.
Latest Posts
Latest Posts
-
Motion Of Molecules Compared To Direction Of Motion Electromagnetic Waves
Apr 01, 2025
-
The Variance Is The Square Root Of The Standard Deviation
Apr 01, 2025
-
Easy Way To Find Common Multiples
Apr 01, 2025
-
What Is The Structural And Functional Unit Of The Kidney
Apr 01, 2025
-
Evolutionary Relationships Between Organisms Are Determined By
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about What Elements Are Gases At Room Temperature . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
