In A Chemical Reaction What Are The Reactants And Products
Muz Play
Mar 29, 2025 · 6 min read
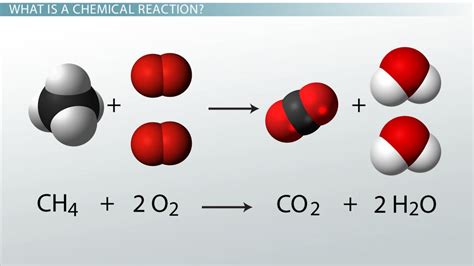
Table of Contents
In a Chemical Reaction: What are Reactants and Products?
Understanding the fundamental concepts of chemical reactions is crucial for anyone venturing into the fascinating world of chemistry. At the heart of every chemical reaction lies a simple yet powerful principle: the transformation of reactants into products. This article delves deep into the definitions, characteristics, and importance of reactants and products, providing a comprehensive guide for both beginners and those seeking a deeper understanding.
Defining Reactants and Products
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. This transformation involves the rearrangement of atoms, resulting in the formation of new substances with different properties. The starting materials in a chemical reaction are called reactants, while the substances formed as a result of the reaction are known as products.
Reactants: The Starting Materials
Reactants are the substances that are initially present and undergo changes during a chemical reaction. They are the ingredients that are mixed together to initiate the reaction. Think of them as the raw materials that are consumed in the process. Reactants can be elements (e.g., hydrogen, oxygen), compounds (e.g., water, carbon dioxide), or a mixture of both. Their chemical identity is altered during the reaction, resulting in the formation of new substances—the products.
Characteristics of Reactants:
- Chemical Composition: Reactants possess a specific chemical formula representing the types and numbers of atoms within their molecules.
- Physical Properties: Reactants have distinct physical properties like color, odor, density, melting point, and boiling point. These properties may change during the reaction.
- Reactivity: The reactivity of a reactant determines its ability to participate in a chemical reaction. Some reactants are highly reactive, while others are less so. This reactivity is influenced by factors such as electronegativity, bond strength, and the presence of catalysts.
- Concentration: The concentration of reactants plays a significant role in the rate of a chemical reaction. Higher concentrations generally lead to faster reaction rates.
Products: The Resulting Substances
Products are the new substances formed as a result of a chemical reaction. They represent the outcome of the transformation of the reactants. The chemical composition and properties of the products are distinct from those of the reactants. Just as reactants can be elements or compounds, so too can products.
Characteristics of Products:
- New Chemical Composition: Products possess a chemical composition different from that of the reactants. This signifies a fundamental change in the arrangement of atoms.
- Different Physical Properties: Products often exhibit physical properties (color, odor, state of matter) that differ significantly from the reactants.
- Formation and Isolation: The products are formed as a consequence of the chemical reaction. Often, the products are isolated and purified from the reaction mixture to determine their identity and properties.
- Yield: The yield of a product refers to the amount of product obtained compared to the theoretical maximum. Factors like reaction conditions and side reactions can influence the yield.
Representing Chemical Reactions: Chemical Equations
Chemical reactions are concisely represented using chemical equations. A chemical equation uses symbols and formulas to show the reactants and products involved in a reaction. The reactants are written on the left side of the equation, while the products are written on the right side. An arrow separates the reactants and products, indicating the direction of the reaction.
For example, the reaction between hydrogen gas and oxygen gas to produce water is represented as follows:
2H₂ + O₂ → 2H₂O
In this equation:
- 2H₂ and O₂ are the reactants.
- 2H₂O is the product.
- The coefficients (2 and 2) represent the stoichiometric ratios, indicating the relative number of molecules of each substance involved in the reaction.
Types of Chemical Reactions
Chemical reactions are classified into various types based on the changes occurring during the reaction. Some common types include:
- Synthesis (Combination) Reactions: Two or more reactants combine to form a single product. Example: A + B → AB
- Decomposition Reactions: A single reactant breaks down into two or more simpler products. Example: AB → A + B
- Single Displacement (Substitution) Reactions: One element replaces another element in a compound. Example: A + BC → AC + B
- Double Displacement (Metathesis) Reactions: Two compounds exchange ions to form two new compounds. Example: AB + CD → AD + CB
- Combustion Reactions: A substance reacts rapidly with oxygen, often producing heat and light. Example: CH₄ + 2O₂ → CO₂ + 2H₂O
- Acid-Base Reactions (Neutralization): An acid reacts with a base to form salt and water. Example: HCl + NaOH → NaCl + H₂O
- Redox Reactions (Oxidation-Reduction): Reactions involving the transfer of electrons between reactants. One reactant undergoes oxidation (loss of electrons), while the other undergoes reduction (gain of electrons).
Factors Affecting Chemical Reactions
Several factors can influence the rate and outcome of a chemical reaction:
- Temperature: Increasing temperature generally increases the rate of reaction.
- Concentration: Higher reactant concentrations lead to faster reaction rates.
- Pressure: Pressure primarily affects gaseous reactions. Increased pressure usually increases the rate of reaction.
- Surface Area: For reactions involving solids, a larger surface area increases the rate of reaction.
- Catalysts: Catalysts are substances that increase the rate of a reaction without being consumed themselves.
- Inhibitors: Inhibitors are substances that slow down the rate of a reaction.
Importance of Reactants and Products
Understanding reactants and products is fundamental to various aspects of chemistry and its applications:
- Industrial Processes: Chemical reactions are the basis of numerous industrial processes, from manufacturing plastics and fertilizers to producing pharmaceuticals and fuels. Careful control of reactants and their reaction conditions is crucial for optimizing product yield and quality.
- Environmental Chemistry: Understanding chemical reactions helps in analyzing environmental issues, such as pollution and remediation. Identifying reactants and their transformation into products is essential for developing strategies for environmental protection.
- Biological Systems: Biological systems are complex networks of chemical reactions. The reactants and products in these reactions are essential for life processes, including metabolism, energy production, and growth.
- Analytical Chemistry: Analytical chemists use chemical reactions to identify and quantify substances in samples. The analysis of reactants and products is crucial for determining the composition of materials.
- Material Science: Designing new materials with specific properties often involves controlling chemical reactions to produce materials with desired characteristics.
Conclusion: The Dynamic Duo of Chemical Reactions
Reactants and products are intrinsically linked in the world of chemical reactions. The reactants, the initial ingredients, undergo transformation, leading to the formation of new substances – the products. A comprehensive understanding of their characteristics, interactions, and the factors affecting their behavior is crucial for navigating the complexities of chemical processes. Whether in industrial settings, environmental studies, biological systems, or material science, the interplay of reactants and products forms the bedrock of countless applications, impacting our lives in profound ways. This knowledge empowers us to manipulate and understand the world around us, enabling innovation and addressing challenges across a diverse range of fields. The ongoing exploration of chemical reactions and their dynamics continues to fuel scientific advancement and technological progress.
Latest Posts
Latest Posts
-
How To Find C In A Sinusoidal Function
Apr 01, 2025
-
Extraction And Processing Of Fossil Fuels Quick Check
Apr 01, 2025
-
What Does A Catalytic Converter Turn Nitrogen Oxide Into
Apr 01, 2025
-
Lipids Hate Water And Are Said To Be
Apr 01, 2025
-
Abstract Algebra Theory And Applications Judson
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about In A Chemical Reaction What Are The Reactants And Products . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
