What Does A Catalytic Converter Turn Nitrogen Oxide Into
Muz Play
Apr 01, 2025 · 6 min read
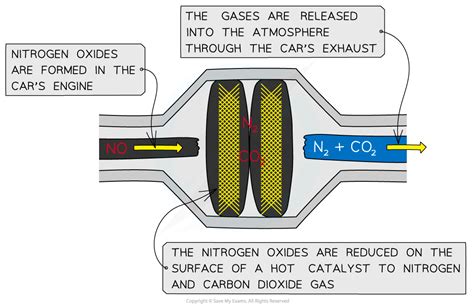
Table of Contents
What Does a Catalytic Converter Turn Nitrogen Oxides Into? Understanding Emission Control
The catalytic converter, a vital component of modern vehicles' exhaust systems, plays a crucial role in reducing harmful emissions. While many understand its function in general terms, the specifics of its chemical processes, particularly regarding nitrogen oxides (NOx), often remain unclear. This article delves deep into the fascinating chemistry within a catalytic converter, focusing specifically on how it transforms harmful nitrogen oxides into less harmful substances. We’ll explore the different types of catalytic converters, the science behind the reactions, and the environmental significance of this transformative process.
Understanding Nitrogen Oxides (NOx) and Their Environmental Impact
Before delving into the catalytic converter's function, let's establish a clear understanding of nitrogen oxides (NOx). This term encompasses a group of highly reactive gases containing nitrogen and oxygen, primarily nitric oxide (NO) and nitrogen dioxide (NO2). These gases are formed during high-temperature combustion processes, such as those occurring within a vehicle's internal combustion engine.
The harmful effects of NOx are significant:
- Respiratory problems: NOx irritates the lungs and airways, exacerbating conditions like asthma and bronchitis. Long-term exposure can lead to more serious respiratory illnesses.
- Acid rain: NOx reacts with water vapor in the atmosphere to form nitric acid, a major component of acid rain. Acid rain damages ecosystems, buildings, and infrastructure.
- Smog formation: NOx contributes significantly to the formation of photochemical smog, a haze of pollutants that reduces visibility and poses health risks.
- Ozone depletion: While less directly involved than chlorofluorocarbons (CFCs), NOx can indirectly contribute to ozone depletion in the stratosphere.
The Catalytic Converter: A Chemical Transformer
The catalytic converter is strategically positioned in the exhaust system, downstream from the engine. Its primary function is to chemically transform harmful exhaust gases, including NOx, carbon monoxide (CO), and unburnt hydrocarbons (HC), into less harmful substances.
This transformation occurs through a series of complex oxidation and reduction reactions facilitated by a catalyst. The catalyst typically consists of precious metals, such as platinum, palladium, and rhodium, finely dispersed on a ceramic or metallic substrate. This high surface area maximizes contact between the catalyst and the exhaust gases, increasing the efficiency of the conversion process.
How the Catalytic Converter Handles Nitrogen Oxides
The catalytic converter's action on NOx involves a complex interplay of oxidation and reduction reactions, depending on the type of converter:
1. Three-Way Catalytic Converters (TWCs)
The most common type of catalytic converter is the three-way catalytic converter (TWC). As the name suggests, it simultaneously addresses three pollutants: NOx, CO, and HC. To effectively tackle NOx, TWCs utilize a carefully balanced air-fuel mixture (stoichiometric ratio). This precise balance ensures that sufficient oxygen is present to oxidize CO and HC, while simultaneously providing the right conditions to reduce NOx.
The key reaction within a TWC for NOx reduction is:
2NO + 2CO → N₂ + 2CO₂
In this reaction, nitric oxide (NO) reacts with carbon monoxide (CO) in the presence of the catalyst to produce nitrogen gas (N₂) and carbon dioxide (CO₂). Nitrogen gas is a relatively inert and harmless component of the atmosphere, while carbon dioxide, although a greenhouse gas, is significantly less harmful than NOx.
Another significant NOx reduction reaction in the TWC is:
2NO₂ + 4CO → N₂ + 4CO₂
This reaction shows the reduction of nitrogen dioxide (NO2), another component of NOx, to nitrogen gas and carbon dioxide.
2. Selective Catalytic Reduction (SCR) Systems
While TWCs are highly effective, they are most efficient under stoichiometric conditions. Lean-burn engines, designed for improved fuel efficiency, operate with an excess of oxygen, creating a less favorable environment for NOx reduction in a TWC. This is where Selective Catalytic Reduction (SCR) systems come into play.
SCR systems use a separate catalyst and a reducing agent, typically urea (automotive-grade diesel exhaust fluid or DEF), to selectively reduce NOx. The urea decomposes to form ammonia (NH₃), which then reacts with NOx in the presence of the SCR catalyst.
The primary reaction in SCR is:
4NO + 4NH₃ + O₂ → 4N₂ + 6H₂O
This reaction converts NOx into harmless nitrogen gas (N₂) and water (H₂O). SCR systems are particularly effective in reducing NOx emissions from diesel engines, where high levels of NOx are often generated.
3. NOx Storage and Reduction (NSR) Catalysts
Another approach to NOx control, particularly in gasoline engines, involves NOx storage and reduction (NSR) catalysts. These catalysts employ specific materials that can store NOx under oxygen-rich conditions. When the stored NOx reaches a certain level, the system switches to a fuel-rich condition, reducing the stored NOx. This switching between oxygen-rich and fuel-rich conditions allows for effective NOx reduction even in lean-burn engines. The reduction reaction here is similar to those in TWCs, resulting in nitrogen gas and water.
Factors Affecting Catalytic Converter Efficiency
Several factors can influence the efficiency of a catalytic converter in converting nitrogen oxides:
- Catalyst condition: Over time, the catalyst can become poisoned or degraded by contaminants in the exhaust stream, such as sulfur compounds or lead. This reduces its effectiveness in converting NOx.
- Temperature: The catalytic reactions are temperature-dependent. The converter needs to reach a certain operating temperature before it becomes fully active. Cold starts can lead to reduced efficiency initially.
- Air-fuel ratio: As mentioned, the effectiveness of TWCs is highly sensitive to the air-fuel ratio. Deviations from the stoichiometric ratio can significantly reduce their NOx conversion efficiency.
- Exhaust gas composition: The presence of other substances in the exhaust gas, besides NOx, CO, and HC, can affect the catalytic reactions and the overall efficiency of the converter.
Environmental Significance of NOx Reduction
The conversion of nitrogen oxides into less harmful substances by catalytic converters has profound environmental consequences. By reducing NOx emissions from vehicles, we see improvements in:
- Air quality: Cleaner air leads to reduced respiratory illnesses and improved public health.
- Acid rain mitigation: Less NOx means less nitric acid formation and reduced acid rain damage.
- Smog reduction: Decreased NOx contributes to less smog formation, leading to improved visibility and reduced respiratory problems associated with smog.
- Protection of ecosystems: Reduced acid rain and air pollution help protect sensitive ecosystems and biodiversity.
Conclusion: The Ongoing Evolution of Emission Control
The catalytic converter's role in transforming nitrogen oxides into harmless substances is a critical advancement in emission control technology. While TWCs remain prevalent, advancements in SCR and NSR systems continue to refine emission control strategies, particularly for lean-burn engines. Understanding the chemistry behind these processes highlights the importance of ongoing research and development in improving air quality and protecting the environment. Future developments will likely focus on even more efficient and durable catalysts, along with further integration of advanced emission control technologies to minimize the environmental impact of vehicles. The continuous evolution of these technologies underlines the commitment to cleaner air and a healthier planet.
Latest Posts
Latest Posts
-
What Is The Difference Between Ethnic And Religious Groups
Apr 02, 2025
-
Explain The Difference Between An Autotroph And A Heterotroph
Apr 02, 2025
-
Is Magnesium A Metal Nonmetal Or Metalloid
Apr 02, 2025
-
Number Of Atoms In A Simple Cubic Unit Cell
Apr 02, 2025
-
Inscribed Circle In A Right Triangle
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about What Does A Catalytic Converter Turn Nitrogen Oxide Into . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
