How Do You Know If A Reaction Is Spontaneous
Muz Play
Mar 24, 2025 · 5 min read
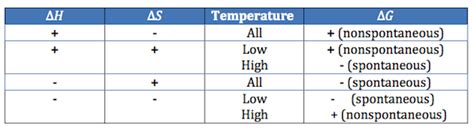
Table of Contents
- How Do You Know If A Reaction Is Spontaneous
- Table of Contents
- How Do You Know if a Reaction is Spontaneous?
- The Role of Enthalpy (ΔH)
- Examples of Enthalpy-Driven Reactions:
- The Role of Entropy (ΔS)
- Examples of Entropy-Driven Reactions:
- Gibbs Free Energy (ΔG) - The Ultimate Determinant of Spontaneity
- Understanding the interplay of ΔH, ΔS, and T:
- Practical Applications and Considerations
- Standard Free Energy Change (ΔG°)
- Non-Standard Conditions
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
How Do You Know if a Reaction is Spontaneous?
Understanding spontaneity in chemical reactions is crucial for predicting whether a process will occur naturally without external intervention. This seemingly simple concept delves into the intricate world of thermodynamics, encompassing enthalpy, entropy, and Gibbs Free Energy. This comprehensive guide will explore the various ways to determine if a reaction is spontaneous, explaining the underlying principles and providing practical examples.
The Role of Enthalpy (ΔH)
Enthalpy, denoted as ΔH, measures the heat change during a reaction at constant pressure. A negative ΔH indicates an exothermic reaction, where heat is released to the surroundings. These reactions are often, but not always, spontaneous. Think of combustion – burning wood releases heat and proceeds spontaneously.
A positive ΔH signifies an endothermic reaction, where heat is absorbed from the surroundings. Endothermic reactions typically require an energy input to proceed and are generally not spontaneous under standard conditions. Melting ice, for instance, requires heat input and isn't spontaneous at temperatures below 0°C.
Examples of Enthalpy-Driven Reactions:
- Combustion: The burning of fuels like methane (CH₄) or propane (C₃H₈) releases significant heat, making them highly spontaneous.
- Neutralization Reactions: The reaction between an acid and a base, such as hydrochloric acid (HCl) and sodium hydroxide (NaOH), generates heat and is spontaneous.
- Formation of many ionic compounds: The formation of salt crystals from their constituent ions in solution is often exothermic and therefore spontaneous.
The Role of Entropy (ΔS)
Entropy, denoted as ΔS, measures the degree of disorder or randomness in a system. A positive ΔS indicates an increase in disorder, meaning the products are more disordered than the reactants. An increase in entropy favors spontaneity.
A negative ΔS indicates a decrease in disorder, meaning the products are more ordered than the reactants. A decrease in entropy works against spontaneity.
Consider the melting of ice: the liquid water (product) is more disordered than the solid ice (reactant), resulting in a positive ΔS. This increase in entropy contributes to the spontaneity of melting at temperatures above 0°C. Conversely, the formation of a crystal from a solution shows a decrease in entropy (negative ΔS) as the ordered crystal structure forms.
Examples of Entropy-Driven Reactions:
- Dissolution of solids in liquids: The solid becomes dispersed in the liquid, increasing the disorder, hence a positive ΔS.
- Evaporation of liquids: Liquids transition to the gaseous phase, significantly increasing molecular randomness (positive ΔS).
- Many chemical reactions involving the production of gases: Gas molecules have much greater freedom of movement than liquids or solids, resulting in a substantial increase in entropy.
Gibbs Free Energy (ΔG) - The Ultimate Determinant of Spontaneity
While enthalpy and entropy provide valuable insights, the Gibbs Free Energy (ΔG) is the ultimate criterion for determining spontaneity under constant temperature and pressure. It combines both enthalpy and entropy:
ΔG = ΔH - TΔS
where:
- ΔG = Gibbs Free Energy change
- ΔH = Enthalpy change
- T = Absolute temperature (in Kelvin)
- ΔS = Entropy change
The sign of ΔG dictates the spontaneity:
- ΔG < 0 (negative): The reaction is spontaneous under the given conditions.
- ΔG > 0 (positive): The reaction is non-spontaneous under the given conditions. It requires energy input to proceed.
- ΔG = 0 (zero): The reaction is at equilibrium; there is no net change in the concentrations of reactants and products.
Understanding the interplay of ΔH, ΔS, and T:
The temperature (T) plays a critical role in determining spontaneity. Let's analyze the different scenarios:
- ΔH < 0 and ΔS > 0: This combination always results in ΔG < 0 (spontaneous at all temperatures). This is the ideal scenario – an exothermic reaction with an increase in entropy.
- ΔH > 0 and ΔS < 0: This combination always results in ΔG > 0 (non-spontaneous at all temperatures). This is unfavorable – an endothermic reaction with a decrease in entropy.
- ΔH < 0 and ΔS < 0: ΔG depends on the temperature. At low temperatures, the negative ΔH term dominates, making ΔG negative (spontaneous). At high temperatures, the TΔS term can become larger than the ΔH term, making ΔG positive (non-spontaneous).
- ΔH > 0 and ΔS > 0: ΔG depends on the temperature. At low temperatures, the positive ΔH term dominates, making ΔG positive (non-spontaneous). At high temperatures, the TΔS term can outweigh the ΔH term, resulting in ΔG becoming negative (spontaneous).
Practical Applications and Considerations
The principles of spontaneity are applied extensively in various fields:
- Chemistry: Predicting the feasibility of chemical reactions, designing efficient synthesis pathways.
- Materials Science: Developing new materials with desired properties, understanding phase transitions.
- Biology: Studying metabolic processes, understanding enzyme activity, and predicting protein folding.
- Environmental Science: Assessing the environmental impact of chemical reactions, predicting pollutant behavior.
Standard Free Energy Change (ΔG°)
The standard free energy change (ΔG°) refers to the free energy change under standard conditions (298 K and 1 atm pressure). It provides a benchmark for comparing the spontaneity of different reactions. However, it's crucial to remember that standard conditions might not always reflect real-world scenarios.
Non-Standard Conditions
In many real-world situations, reactions occur under non-standard conditions. To determine spontaneity under these conditions, we use the following equation:
ΔG = ΔG° + RTlnQ
where:
- R is the gas constant
- T is the temperature in Kelvin
- Q is the reaction quotient (the ratio of product and reactant activities at a given point)
This equation accounts for deviations from standard conditions based on the actual concentrations of reactants and products.
Conclusion
Determining if a reaction is spontaneous involves a multifaceted analysis encompassing enthalpy, entropy, and the interplay of both factors through Gibbs Free Energy. While enthalpy and entropy provide valuable clues, Gibbs Free Energy is the definitive measure, especially when considering the effect of temperature and non-standard conditions. Understanding these concepts is critical for comprehending the driving forces behind chemical and physical processes and allows for predicting the behavior of systems under various conditions. This knowledge is essential across diverse scientific and engineering disciplines, highlighting the importance of thermodynamics in understanding the world around us.
Latest Posts
Latest Posts
-
How Many People Are In A Group
Mar 27, 2025
-
Example Of A Line In Poetry
Mar 27, 2025
-
How To Read A Gc Chromatogram
Mar 27, 2025
-
How To Find Domain Of Vector Function
Mar 27, 2025
-
In Which Circumstance Would The Courts Find Libel
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How Do You Know If A Reaction Is Spontaneous . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
