How Does Pressure Affect The Equilibrium
Muz Play
Mar 23, 2025 · 5 min read
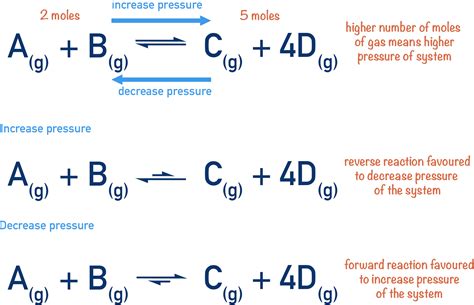
Table of Contents
How Does Pressure Affect Equilibrium? A Comprehensive Guide
Understanding how pressure affects chemical equilibrium is crucial in chemistry. This detailed guide explores the intricacies of Le Chatelier's principle as it relates to pressure changes, providing clear explanations and examples to help you master this concept.
Le Chatelier's Principle: The Foundation
At the heart of understanding pressure's effect on equilibrium lies Le Chatelier's principle. This principle states that if a change of condition is applied to a system in equilibrium, the system will shift in a direction that relieves the stress. In simpler terms, the system will adjust to counteract the change imposed upon it. This is particularly relevant when discussing changes in pressure.
Pressure Changes and Equilibrium Shifts: The Gas Phase
Pressure changes significantly impact equilibrium only when gaseous reactants or products are involved. This is because pressure directly relates to the concentration of gases. An increase in pressure increases the concentration of gaseous molecules, while a decrease in pressure has the opposite effect.
Think of it this way: Imagine a container filled with gas molecules. Compressing the container (increasing pressure) forces more molecules into a smaller space, thus increasing their concentration. Expanding the container (decreasing pressure) has the opposite effect.
Predicting Equilibrium Shifts with Pressure Changes
To predict how a pressure change will affect equilibrium, follow these steps:
- Identify the gaseous species: Determine which reactants and products are in the gaseous phase.
- Count the moles of gas: Determine the total number of moles of gas on the reactant side and the total number of moles of gas on the product side.
- Apply Le Chatelier's principle: A pressure increase favors the side with fewer moles of gas, while a pressure decrease favors the side with more moles of gas.
Let's illustrate this with examples:
Example 1: The Haber-Bosch Process
The Haber-Bosch process, used to synthesize ammonia (NH₃), is a prime example:
N₂(g) + 3H₂(g) ⇌ 2NH₃(g)
In this reaction:
-
Reactant side: 4 moles of gas (1 mole N₂ + 3 moles H₂)
-
Product side: 2 moles of gas (2 moles NH₃)
-
Effect of increased pressure: Increasing the pressure will shift the equilibrium to the right, favoring the production of ammonia (fewer moles of gas). This is because the system relieves the stress (increased pressure) by reducing the number of gas molecules.
-
Effect of decreased pressure: Decreasing the pressure will shift the equilibrium to the left, favoring the production of nitrogen and hydrogen (more moles of gas). This is because the system relieves the stress (decreased pressure) by increasing the number of gas molecules.
Example 2: The Decomposition of Calcium Carbonate
The decomposition of calcium carbonate is another illustrative example:
CaCO₃(s) ⇌ CaO(s) + CO₂(g)
In this reaction:
-
Reactant side: 0 moles of gas
-
Product side: 1 mole of gas (CO₂)
-
Effect of increased pressure: Increasing the pressure will shift the equilibrium to the left, favoring the formation of calcium carbonate. The system relieves the stress by reducing the amount of gaseous CO₂.
-
Effect of decreased pressure: Decreasing the pressure will shift the equilibrium to the right, favoring the decomposition of calcium carbonate and producing more CO₂ gas. This increases the number of gas molecules, relieving the stress of decreased pressure.
The Role of Catalysts
It's crucial to remember that catalysts do not affect the position of equilibrium. While catalysts speed up the rate at which equilibrium is reached, they do not alter the equilibrium concentrations of reactants and products. They simply provide an alternative reaction pathway with a lower activation energy.
The Importance of Temperature
While this article focuses on pressure, it's important to note that temperature also significantly impacts equilibrium. Changes in temperature affect the equilibrium constant (K), which in turn determines the equilibrium concentrations. Unlike pressure, the effect of temperature on equilibrium isn't easily predicted without knowing the enthalpy change (ΔH) of the reaction. Exothermic reactions (ΔH < 0) are favored at lower temperatures, while endothermic reactions (ΔH > 0) are favored at higher temperatures.
Real-World Applications
The principles discussed here have numerous real-world applications:
- Industrial processes: The Haber-Bosch process, mentioned earlier, is a cornerstone of industrial nitrogen fixation, vital for fertilizer production. Understanding the pressure's effect is essential for optimizing ammonia yield.
- Environmental science: Equilibrium principles are crucial in understanding atmospheric processes, such as the formation of smog or the distribution of gases in the atmosphere.
- Geochemistry: Pressure plays a key role in geological formations and mineral equilibria deep within the earth's crust.
Beyond Simple Cases: More Complex Equilibria
The examples above involve relatively simple equilibria. More complex scenarios might involve multiple gaseous species or reactions occurring simultaneously. In such cases, careful consideration of all gaseous components and their stoichiometric coefficients is necessary to predict the equilibrium shift accurately.
Conclusion: Mastering Equilibrium Shifts
Understanding how pressure affects equilibrium is fundamental to mastering chemical thermodynamics. By applying Le Chatelier's principle and carefully analyzing the number of gas molecules on both sides of the reaction, you can accurately predict the direction of equilibrium shifts in response to pressure changes. This understanding has profound implications in various scientific and industrial fields, highlighting the importance of this concept. Remember to also consider temperature's impact and the role of catalysts for a complete understanding of equilibrium dynamics. Further exploration into the mathematical aspects of equilibrium, involving equilibrium constants and partial pressures, will provide even deeper insights into these vital chemical processes. Through consistent study and application of these principles, you'll confidently navigate the complexities of chemical equilibrium and its responsiveness to external factors.
Latest Posts
Latest Posts
-
What Is The Ph Of Neutral Solution
Mar 25, 2025
-
What Is Is Revenue Control Merchant On Credit Card Statement
Mar 25, 2025
-
Positive And Decreasing Rate Of Change
Mar 25, 2025
-
How To Show F Is One To One
Mar 25, 2025
-
Are Aldehydes Or Ketones More Reactive
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about How Does Pressure Affect The Equilibrium . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
