How Does Temperature Relate To Kinetic Energy
Muz Play
Mar 30, 2025 · 5 min read
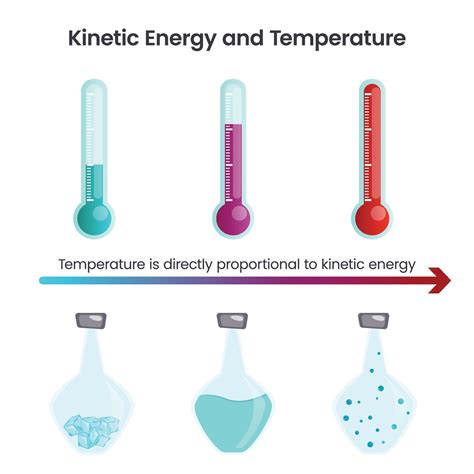
Table of Contents
How Does Temperature Relate to Kinetic Energy? A Deep Dive
The seemingly simple relationship between temperature and kinetic energy underpins much of our understanding of the physical world. From the boiling of water to the movement of stars, this connection is fundamental. But how exactly does temperature relate to kinetic energy? This article will explore this relationship in detail, delving into the microscopic world of atoms and molecules to illuminate the macroscopic effects we observe every day.
Understanding Kinetic Energy
Before exploring the connection between temperature and kinetic energy, let's define kinetic energy itself. Kinetic energy is the energy an object possesses due to its motion. A stationary object has zero kinetic energy. The faster an object moves, the greater its kinetic energy. Quantitatively, kinetic energy (KE) is expressed as:
KE = 1/2 * mv²
Where:
- m represents the mass of the object
- v represents the velocity (speed and direction) of the object
This formula applies to macroscopic objects like cars and planets. However, the concept also extends to the microscopic world of atoms and molecules.
The Microscopic World: Atoms and Molecules in Motion
The world around us is composed of countless atoms and molecules, constantly in motion. This motion is not always visible to the naked eye, but it's always present. Even in seemingly solid objects, atoms vibrate and oscillate around their equilibrium positions. In liquids and gases, this motion is far more pronounced, with molecules moving freely and colliding with each other and the container walls.
This constant, random motion of atoms and molecules is the key to understanding the connection with temperature.
Temperature: A Measure of Average Kinetic Energy
Temperature is not a direct measure of the total kinetic energy of a system. Instead, temperature is a measure of the average kinetic energy of the particles (atoms and molecules) within a system. This crucial distinction highlights the statistical nature of temperature.
Imagine a container filled with gas molecules. These molecules are moving at various speeds, some faster, some slower. The temperature of the gas is directly proportional to the average kinetic energy of these molecules. A higher temperature indicates a higher average kinetic energy, meaning the molecules are, on average, moving faster. Conversely, a lower temperature signifies a lower average kinetic energy, and slower molecular motion.
The Role of Absolute Zero
This relationship leads to the concept of absolute zero, the theoretical temperature at which all molecular motion ceases. At absolute zero (0 Kelvin or -273.15°C), the average kinetic energy of the particles is zero. It's important to note that even at absolute zero, there is still some residual quantum mechanical energy, but the classical concept of kinetic energy is effectively zero.
The Relationship at Different States of Matter
The relationship between temperature and kinetic energy manifests differently in various states of matter:
Solids
In solids, atoms and molecules are tightly bound together. While they don't move freely, they still vibrate around their fixed positions. As temperature increases, the amplitude of these vibrations increases, leading to a higher average kinetic energy. This increased vibrational energy can eventually overcome the intermolecular forces, leading to a phase transition (melting) to a liquid state.
Liquids
In liquids, molecules have more freedom of movement than in solids. They can slide past each other, leading to fluidity. As temperature increases, the average kinetic energy of the molecules rises, leading to increased movement and reduced viscosity. Sufficiently high kinetic energy can lead to the liquid transitioning to a gas (boiling).
Gases
In gases, molecules are far apart and move relatively freely. Their kinetic energy is directly proportional to their temperature. As temperature increases, the molecules move faster, leading to increased pressure if the volume is constant. This is described by the ideal gas law:
PV = nRT
Where:
- P is pressure
- V is volume
- n is the number of moles of gas
- R is the ideal gas constant
- T is temperature in Kelvin
This law highlights the direct relationship between temperature and the average kinetic energy of gas molecules. Increased temperature leads to increased pressure (if volume is constant) because the faster-moving molecules collide more frequently and forcefully with the container walls.
Beyond the Ideal Gas Law: Real-World Considerations
While the ideal gas law provides a good approximation, it assumes certain simplifications that don't always hold true in the real world. Real gases deviate from ideal behavior, particularly at high pressures and low temperatures. Intermolecular forces become more significant under these conditions, affecting the average kinetic energy and thus the pressure.
Applications of the Temperature-Kinetic Energy Relationship
The relationship between temperature and kinetic energy has numerous practical applications:
-
Thermodynamics: Understanding this relationship is crucial in thermodynamics, the study of heat and energy transfer. Engines, refrigerators, and power plants all rely on the principles of heat transfer and the conversion of thermal energy (related to temperature) into mechanical work (related to kinetic energy).
-
Chemistry: Chemical reactions are often temperature-dependent. Higher temperatures increase the average kinetic energy of reactant molecules, leading to more frequent and energetic collisions, thus increasing the reaction rate.
-
Materials Science: The properties of materials are heavily influenced by the kinetic energy of their constituent atoms and molecules. For example, the strength and ductility of metals depend on the vibrations and interactions of their atoms.
-
Meteorology: Understanding the kinetic energy of air molecules is essential for weather prediction. Wind speed, temperature gradients, and atmospheric pressure are all interconnected and depend on the average kinetic energy of air particles.
-
Astronomy: The movement of celestial bodies, from planets orbiting stars to stars moving within galaxies, is governed by principles of gravity and kinetic energy. The temperature of stars is directly related to the kinetic energy of the particles within them, influencing their luminosity and lifespan.
Conclusion: A Fundamental Connection
The relationship between temperature and kinetic energy is a fundamental concept in physics and has far-reaching implications across numerous scientific disciplines. While the ideal gas law provides a simplified model, the underlying principle – that temperature is a measure of the average kinetic energy of particles – remains central to our understanding of the physical world. This connection provides a powerful framework for explaining various phenomena, from the everyday boiling of water to the vast movements of celestial bodies. By understanding this deep connection, we can better grasp and manipulate the physical world around us. Further exploration into advanced topics like statistical mechanics can provide an even deeper understanding of this fundamental relationship.
Latest Posts
Latest Posts
-
Find An Equation For The Tangent Plane To The Surface
Apr 01, 2025
-
All Regions Of Earth Where Organisms Live
Apr 01, 2025
-
Active Sites On The Actin Become Available For Binding After
Apr 01, 2025
-
Is Sodium Methoxide A Strong Nucleophile
Apr 01, 2025
-
Which Statement About Immigration Federalism Is False
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about How Does Temperature Relate To Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
