How Does The Shape Of The Molecule Affect Its Polarity
Muz Play
Mar 18, 2025 · 5 min read
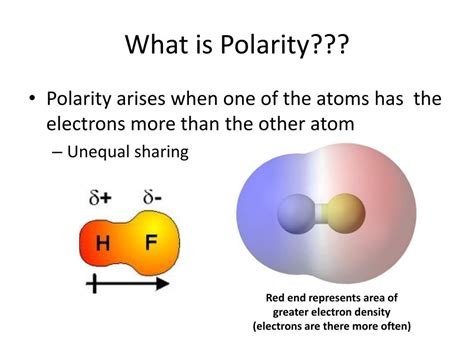
Table of Contents
How Does the Shape of a Molecule Affect its Polarity?
Molecular polarity, a crucial property influencing a molecule's physical and chemical behavior, is significantly impacted by its three-dimensional shape. Understanding this relationship is fundamental to comprehending diverse phenomena, from solubility and boiling points to reactivity and biological interactions. This article delves deep into the intricate connection between molecular geometry and polarity, exploring various factors and providing illustrative examples.
Understanding Polarity: A Quick Recap
Before diving into the influence of molecular shape, let's briefly review the concept of molecular polarity. Polarity arises from the uneven distribution of electron density within a molecule. This uneven distribution is caused by differences in the electronegativity of the constituent atoms. Electronegativity is a measure of an atom's ability to attract electrons towards itself in a chemical bond.
When atoms with significantly different electronegativities bond, the more electronegative atom pulls the shared electrons closer, creating a partial negative charge (δ-) around itself and leaving the less electronegative atom with a partial positive charge (δ+). This creates a dipole moment, a vector quantity representing the magnitude and direction of the charge separation.
A molecule is considered polar if it possesses a net dipole moment, meaning the individual bond dipoles don't cancel each other out. Conversely, a molecule is nonpolar if its bond dipoles cancel due to symmetry.
The Crucial Role of Molecular Geometry
Molecular geometry, the three-dimensional arrangement of atoms in a molecule, plays a pivotal role in determining whether the individual bond dipoles cancel each other out, thus dictating the overall polarity of the molecule. Let's explore how different molecular geometries influence polarity:
1. Linear Molecules
Linear molecules, like carbon dioxide (CO₂), have atoms arranged in a straight line. In CO₂, the two C=O bonds are polar, with oxygen being more electronegative than carbon. However, because the molecule is linear and symmetrical, the two bond dipoles are equal in magnitude and point in opposite directions, resulting in net cancellation. Therefore, CO₂ is a nonpolar molecule, despite having polar bonds.
2. Bent Molecules
Bent molecules, such as water (H₂O), have a slightly bent shape due to the presence of lone pairs of electrons on the central atom (oxygen). The O-H bonds are polar, with oxygen being more electronegative than hydrogen. In this case, the bond dipoles do not cancel each other out because the molecule is bent. The resulting net dipole moment makes water a polar molecule. The presence of lone pairs significantly influences the molecular geometry and consequently the polarity.
3. Trigonal Planar Molecules
Trigonal planar molecules, like boron trifluoride (BF₃), have atoms arranged in a flat, triangular shape. The B-F bonds are polar. However, due to the symmetrical arrangement of the three bonds, the individual bond dipoles cancel each other out, making BF₃ a nonpolar molecule.
4. Tetrahedral Molecules
Tetrahedral molecules, like methane (CH₄), have a central atom surrounded by four atoms arranged at the corners of a tetrahedron. The C-H bonds are relatively nonpolar due to the small electronegativity difference between carbon and hydrogen. Even if a small dipole were present in each bond, the symmetrical tetrahedral arrangement ensures complete cancellation, rendering CH₄ a nonpolar molecule.
5. Trigonal Pyramidal Molecules
Trigonal pyramidal molecules, like ammonia (NH₃), have a central atom bonded to three other atoms, with one lone pair of electrons. The N-H bonds are polar. The presence of the lone pair distorts the geometry, preventing the bond dipoles from completely canceling each other out. This asymmetry leads to a net dipole moment, making ammonia a polar molecule.
Factors Affecting Molecular Shape and Polarity
Several factors influence the molecular shape and subsequently its polarity:
-
Number of electron pairs: The number of electron pairs (both bonding and non-bonding) around the central atom determines the basic geometry according to the Valence Shell Electron Pair Repulsion (VSEPR) theory. Lone pairs exert stronger repulsive forces than bonding pairs, influencing the bond angles and overall shape.
-
Bonding pairs vs. lone pairs: The presence and position of lone pairs significantly impact molecular geometry. Lone pairs occupy more space than bonding pairs, leading to deviations from ideal geometries and affecting dipole moment cancellation.
-
Electronegativity difference: The greater the electronegativity difference between the bonded atoms, the more polar the individual bonds become. This directly influences the magnitude of the dipole moment.
-
Bond length: While less significant than electronegativity difference, bond length also plays a subtle role. Longer bonds generally result in smaller dipole moments.
Examples and Implications
Understanding the relationship between molecular shape and polarity is crucial in explaining various properties and phenomena:
-
Solubility: Polar molecules generally dissolve well in polar solvents (like water), while nonpolar molecules dissolve better in nonpolar solvents (like hexane). This is due to the "like dissolves like" principle.
-
Boiling points: Polar molecules generally have higher boiling points than nonpolar molecules of comparable molecular weight due to stronger intermolecular forces (dipole-dipole interactions and hydrogen bonding).
-
Surface tension: Polar molecules often exhibit higher surface tension than nonpolar molecules due to strong intermolecular attractions.
-
Reactivity: Molecular polarity influences reactivity, as polar molecules often participate in reactions involving charge interactions.
-
Biological activity: Polarity is vital in biological systems, impacting the interactions of molecules with biological membranes and receptors. For instance, the polarity of water is crucial for its role as a solvent and in many biological processes.
Beyond Simple Molecules: More Complex Scenarios
The relationship between molecular shape and polarity becomes more complex in larger molecules with multiple polar bonds and diverse functional groups. In these cases, predicting polarity requires a detailed consideration of the three-dimensional structure and the vector addition of individual bond dipoles. Computational methods and molecular modeling techniques are often employed to analyze the polarity of such complex molecules.
Conclusion
The shape of a molecule significantly influences its polarity. Understanding this intricate relationship requires a firm grasp of molecular geometry principles (like VSEPR theory), electronegativity differences, and the impact of lone pairs. This knowledge is fundamental for predicting and understanding a wide range of physical and chemical properties, paving the way for advancements in various scientific disciplines, including chemistry, biology, and materials science. The examples and explanations provided in this article aim to provide a comprehensive understanding of this critical concept, enabling readers to analyze and predict the polarity of diverse molecules. Further exploration into advanced computational techniques and experimental methods can deepen this understanding for more complex molecular systems.
Latest Posts
Latest Posts
-
How Is Atp Made During Fermentation
Mar 18, 2025
-
Is Main A Keyword In Fortran
Mar 18, 2025
-
How To Find Bond Dissociation Energy
Mar 18, 2025
-
Where Does Mrna Go After It Leaves The Nucleus
Mar 18, 2025
-
What Is The Difference Between Primary And Secondary Growth
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Does The Shape Of The Molecule Affect Its Polarity . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
