How Is Temperature Related To Kinetic Energy
Muz Play
Mar 25, 2025 · 5 min read
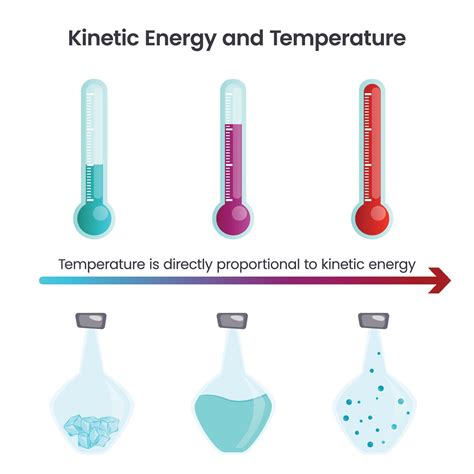
Table of Contents
How is Temperature Related to Kinetic Energy? A Deep Dive
Temperature and kinetic energy are intrinsically linked; understanding their relationship is fundamental to grasping many aspects of physics and chemistry. This article will explore this connection in detail, delving into the microscopic world of atoms and molecules to explain macroscopic phenomena like heat transfer and phase transitions. We'll examine how temperature acts as a measure of average kinetic energy, explore the nuances of different types of kinetic energy within a system, and discuss exceptions and limitations to the general relationship.
The Microscopic Dance: Atoms, Molecules, and Kinetic Energy
At the heart of the matter lies the kinetic theory of gases, which provides a robust framework for understanding the relationship between temperature and kinetic energy. This theory posits that matter is composed of tiny particles – atoms and molecules – that are in constant, random motion. This motion encompasses various forms of kinetic energy:
1. Translational Kinetic Energy:
This is the most straightforward form of kinetic energy, referring to the movement of the entire atom or molecule from one point to another. Imagine a single gas molecule bouncing around in a container – its translational kinetic energy is directly related to its speed and mass:
KE<sub>trans</sub> = ½mv²
where:
- KE<sub>trans</sub> represents translational kinetic energy
- m is the mass of the particle
- v is the velocity of the particle
2. Rotational Kinetic Energy:
Molecules, unlike atoms (which are essentially point masses for our purposes here), can also rotate. This rotational motion contributes to the molecule's overall kinetic energy. The complexity of calculating rotational kinetic energy depends on the molecule's shape and the distribution of its mass. Linear molecules, for example, have simpler rotational behavior than more complex, non-linear molecules.
3. Vibrational Kinetic Energy:
Atoms within a molecule are bonded together, and these bonds aren't rigid. They vibrate, stretching and compressing, contributing to the molecule's total kinetic energy. The vibrational kinetic energy depends on the strength of the chemical bonds and the mass of the atoms involved. More complex molecules have more vibrational modes, increasing their overall vibrational kinetic energy.
Temperature: A Measure of Average Kinetic Energy
Temperature is not a measure of the total kinetic energy of a system, but rather the average kinetic energy of its constituent particles. This distinction is crucial. A system can have a high total kinetic energy due to a large number of particles, even if the average kinetic energy (and thus temperature) is low. Conversely, a small system with high-speed particles can have a high temperature despite a low total kinetic energy.
The relationship between temperature (in Kelvin) and the average translational kinetic energy is given by:
KE<sub>avg</sub> = (3/2)kT
where:
- KE<sub>avg</sub> is the average translational kinetic energy per particle
- k is the Boltzmann constant (a fundamental constant relating temperature to energy)
- T is the absolute temperature in Kelvin
This equation highlights a direct proportionality: as temperature increases, so does the average kinetic energy of the particles. This explains why hotter objects feel warmer – their particles are moving faster, transferring more energy upon contact.
Implications of the Temperature-Kinetic Energy Relationship
This fundamental relationship has far-reaching consequences in various fields:
1. Heat Transfer:
Heat transfer is essentially the flow of energy from a region of higher temperature (higher average kinetic energy) to a region of lower temperature (lower average kinetic energy). This flow continues until thermal equilibrium is reached, where both regions have the same average kinetic energy and therefore the same temperature.
2. Phase Transitions:
Changes in state (solid to liquid, liquid to gas, etc.) are driven by changes in the kinetic energy of the particles. As temperature increases, the average kinetic energy overcomes the intermolecular forces holding particles together, leading to a phase transition. For instance, the melting of ice involves providing enough energy to break the hydrogen bonds holding the water molecules in a fixed crystalline structure.
3. Chemical Reactions:
Chemical reactions often require a certain minimum amount of kinetic energy, called the activation energy, to proceed. Higher temperatures, with their increased average kinetic energy, increase the likelihood of particles colliding with sufficient energy to overcome the activation energy barrier, thus speeding up the reaction rate.
4. Ideal Gas Law:
The ideal gas law, PV = nRT, is a direct consequence of the kinetic theory of gases. The pressure (P) exerted by an ideal gas is directly proportional to the average kinetic energy of its particles, which is, in turn, directly proportional to the temperature (T).
Beyond the Ideal: Deviations and Exceptions
While the relationship between temperature and kinetic energy is generally robust, several factors can cause deviations from the simple model described above:
1. Non-ideal Gases:
At high pressures or low temperatures, real gases deviate from ideal gas behavior. Intermolecular forces, neglected in the ideal gas model, become significant, affecting the relationship between temperature and kinetic energy.
2. Quantum Effects:
At extremely low temperatures, quantum mechanical effects become significant, and the classical kinetic theory breaks down. Particles exhibit wave-like behavior, and their motion is no longer solely governed by classical mechanics.
3. Specific Heat Capacity:
The specific heat capacity of a substance reflects how much energy is needed to raise its temperature by a certain amount. This value depends not only on the average kinetic energy but also on the substance's ability to store energy in other forms, such as vibrational modes. This means that different materials will have varying temperature changes for the same amount of energy added, further complicating the direct relationship.
Conclusion: A Dynamic Relationship
The relationship between temperature and kinetic energy is a cornerstone of our understanding of the physical world. While the simple model of average translational kinetic energy provides a useful framework, it's crucial to acknowledge the complexities introduced by rotational and vibrational energy, non-ideal gas behavior, and quantum effects at extreme temperatures. This intricate relationship drives numerous phenomena, from heat transfer and phase transitions to chemical reactions and the behavior of gases. A deeper understanding of this connection unlocks a deeper understanding of the universe around us. Further exploration into advanced thermodynamics and statistical mechanics will reveal even more subtle aspects of this fundamental relationship.
Latest Posts
Latest Posts
-
The Martian And The Car Answer Key
Mar 27, 2025
-
Rusting Of Iron Chemical Or Physical Change
Mar 27, 2025
-
How Many Neutrons Does K Have
Mar 27, 2025
-
A Larger Nucleus Splits Apart Making 2 Smaller Ones
Mar 27, 2025
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about How Is Temperature Related To Kinetic Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
