How Many Bonds Can Boron Form
Muz Play
Mar 31, 2025 · 5 min read
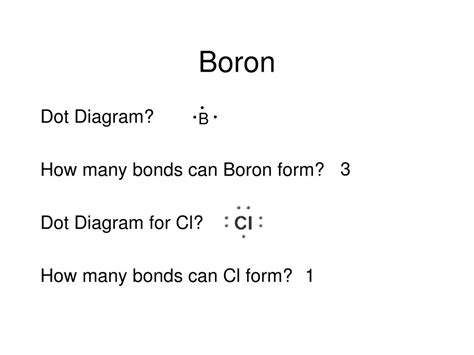
Table of Contents
How Many Bonds Can Boron Form? Exploring the Chemistry of Boron Bonding
Boron, the fifth element on the periodic table, presents a fascinating case study in chemical bonding. Unlike its neighbors carbon and silicon, which consistently form four bonds, boron's bonding behavior is more nuanced and diverse, leading to a complex and interesting area of chemical exploration. This article delves deep into the question: how many bonds can boron form? The answer, as we will see, isn't a simple number, but rather a range influenced by several factors including its electronic configuration, the nature of the bonding partners, and the overall molecular structure.
The Electronic Configuration: The Foundation of Boron's Bonding
Understanding boron's bonding capacity begins with its electronic configuration: 1s²2s²2p¹. With only three valence electrons (in the 2s and 2p orbitals), simple valence bond theory might suggest a maximum of three bonds. However, this simplistic view doesn't fully capture the complexity of boron's chemistry. The ability of boron to utilize its empty 2p orbital in bonding significantly expands its bonding possibilities.
Hypervalency: Expanding Beyond the Octet Rule
While the octet rule (the tendency of atoms to have eight electrons in their valence shell) serves as a useful guideline for many elements, boron frequently exhibits hypovalency (fewer than eight valence electrons) and occasionally demonstrates characteristics of hypervalency in specific bonding situations. This means boron can participate in bonds beyond the simple three predicted by its valence electrons.
Three-Coordinate Boron: The Most Common Scenario
Boron's most common bonding arrangement involves three covalent bonds, leading to a trigonal planar geometry. This is exemplified in compounds like borane (BH₃) and trifluoroborane (BF₃). In these molecules, boron achieves a six-electron valence shell, satisfying a stable electronic configuration. However, this state is electron-deficient, making these compounds Lewis acids. They readily accept electron pairs from Lewis bases to form stable adducts, expanding boron's coordination number.
Examples of Three-Coordinate Boron Compounds:
- Boron trifluoride (BF₃): A powerful Lewis acid, readily reacting with Lewis bases such as ammonia (NH₃) to form adducts.
- Triphenylborane (BPh₃): A common reagent in organic chemistry.
- Borane (BH₃): Highly reactive and rarely found as a monomer; it typically dimerizes to form diborane (B₂H₆).
Four-Coordinate Boron: Beyond the Expected
While three-coordinate boron is prevalent, four-coordinate boron also exists in many important compounds. This occurs when boron accepts a fourth electron pair from a Lewis base, expanding its coordination number to four. This leads to a tetrahedral geometry.
Formation of Four-Coordinate Boron Compounds:
Four-coordinate boron compounds often arise from the interaction of three-coordinate boron compounds with Lewis bases. The Lewis base donates a lone pair of electrons to the empty p-orbital of boron.
Examples of Four-Coordinate Boron Compounds:
- Tetrafluoroborate ion (BF₄⁻): A stable anion found in various salts.
- Tetrahydroborate ion (BH₄⁻): Also known as borohydride, a crucial reducing agent in organic chemistry. The hydrogens in this ion are considered to participate in a 3-centre-2-electron bond. This type of bonding is essential for understanding how Boron can exceed its expected valency.
- Organoboranes with four-coordinate boron: Various organoboranes can feature boron atoms bonded to four carbon atoms or a combination of carbon and other atoms.
Five-Coordinate and Six-Coordinate Boron: Less Common, but Significant
Although less frequent than three- and four-coordinate boron, five- and six-coordinate boron compounds exist, mostly in organometallic complexes and certain solid-state structures. These higher coordination numbers involve more complex bonding interactions, often involving d-orbital participation, which are not as readily available to boron, hence their lower occurrence.
Factors Favoring Higher Coordination Numbers:
- Steric Effects: Bulky ligands can sometimes create space for higher coordination around the boron atom.
- Electronic Effects: The presence of electron-donating ligands can further stabilize higher coordination numbers.
- Crystal Packing Forces: In solid-state structures, crystal packing forces can influence boron's coordination environment.
Delving into Diborane (B₂H₆): A Unique Bonding Example
Diborane, B₂H₆, provides a prime example of boron's ability to engage in unusual bonding arrangements. It features two boron atoms bridged by two hydrogen atoms. This bridging involves three-center, two-electron bonds, a fascinating bonding paradigm where three atoms share two electrons. Each boron is bonded to four atoms (two terminal hydrogen atoms and two bridging hydrogen atoms). Although it is not a simple four-coordinate structure it does illustrate boron exceeding what may initially seem as its bonding limit. The formation of these three-center, two-electron bonds is crucial in understanding higher coordination numbers for boron.
The Role of Hybridization in Boron Bonding
The concept of orbital hybridization is essential for understanding boron's bonding versatility. Boron's valence orbitals (one 2s and three 2p orbitals) can hybridize to form various hybrid orbitals. These hybrids can participate in sigma (σ) and pi (π) bonding, which allows for the formation of different bond arrangements.
- sp² Hybridization: This leads to a trigonal planar geometry, commonly observed in three-coordinate boron compounds.
- sp³ Hybridization: This gives rise to a tetrahedral geometry, prominent in four-coordinate boron compounds.
Conclusion: A Versatile Bonder
In summary, the question "How many bonds can boron form?" doesn't have a single definitive answer. Boron’s bonding behaviour is flexible and context-dependent. While three-coordinate boron is the most common, it can readily form four-coordinate compounds and under certain specific conditions, even participate in higher coordination numbers, defying the simple prediction based solely on its three valence electrons. The ability to engage in three-center two-electron bonds and variations in hybridization further enrich boron’s diverse bonding characteristics. This versatility makes boron a central element in many important compounds with applications spanning various fields, from materials science to medicine. Understanding boron's unique bonding capabilities continues to be an area of active research, revealing its complex and fascinating chemical behavior.
Keywords: Boron, bonding, chemical bonding, coordination number, valence electrons, three-center two-electron bond, hypervalency, hypovalency, Lewis acid, Lewis base, diborane, borane, boron trifluoride, tetrafluoroborate, borohydride, hybridization, sp2 hybridization, sp3 hybridization, organoboranes, organic chemistry, inorganic chemistry.
Latest Posts
Latest Posts
-
A Bronsted Lowry Base Is Defined As
Apr 02, 2025
-
Molecular And Empirical Formula Worksheet With Answers
Apr 02, 2025
-
How To Find The Maclaurin Series
Apr 02, 2025
-
How To Make Normal Probability Plot
Apr 02, 2025
-
Chemical Kinetics Of The Iodine Clock Reaction Lab Report
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about How Many Bonds Can Boron Form . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
