How Many Covalent Bonds Does Oxygen Have
Muz Play
Mar 17, 2025 · 5 min read
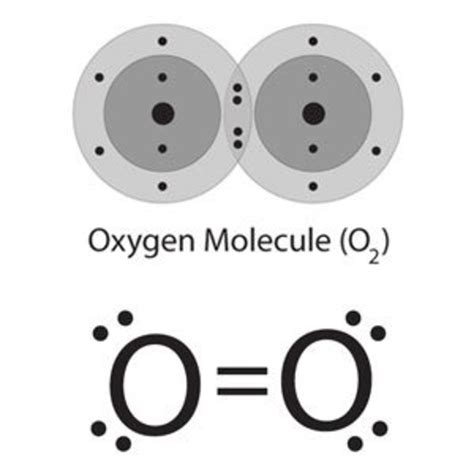
Table of Contents
How Many Covalent Bonds Does Oxygen Have? A Deep Dive into Oxygen's Bonding Behavior
Oxygen, a life-sustaining element crucial for respiration and a major component of water, exhibits fascinating bonding characteristics. Understanding how many covalent bonds oxygen forms is fundamental to comprehending its role in various chemical processes and the properties of the compounds it creates. This article delves into the intricacies of oxygen's bonding behavior, exploring its electronic structure, the formation of covalent bonds, and the exceptions to the general rule.
Oxygen's Electronic Structure: The Foundation of Bonding
To understand oxygen's bonding capacity, we must first examine its electronic structure. Oxygen (O) has an atomic number of 8, meaning it possesses 8 protons and 8 electrons. The electron configuration is 1s²2s²2p⁴. This configuration is crucial because it dictates how oxygen interacts with other atoms to achieve stability.
The Octet Rule and Valence Electrons
The octet rule states that atoms tend to gain, lose, or share electrons to achieve a stable configuration with eight electrons in their outermost shell (valence shell). Oxygen, with six valence electrons (two in the 2s orbital and four in the 2p orbitals), needs two more electrons to complete its octet. This drive to fulfill the octet rule is the primary reason why oxygen forms covalent bonds.
Covalent Bonding in Oxygen: Sharing is Caring
Oxygen achieves a stable octet by sharing electrons with other atoms, a process known as covalent bonding. In a covalent bond, two atoms share one or more pairs of electrons, creating a strong attractive force that holds the atoms together.
Oxygen's Typical Covalent Bonding: Two Bonds
Because oxygen needs two more electrons to complete its octet, it typically forms two covalent bonds. This means it shares two pairs of electrons with other atoms. This pattern is consistently observed in many oxygen-containing compounds.
Examples:
-
Water (H₂O): Oxygen forms two single covalent bonds with two hydrogen atoms. Each hydrogen atom contributes one electron to share with oxygen, completing oxygen's octet and allowing each hydrogen atom to achieve a duet (two electrons in its valence shell).
-
Carbon Dioxide (CO₂): Oxygen forms two double covalent bonds with a single carbon atom. Each oxygen atom shares two pairs of electrons with the carbon atom, resulting in a stable configuration for both oxygen and carbon.
-
Other Oxides: Similar double or single bond formations are typical in various metal and non-metal oxides.
Exceptions to the Rule: The Case of the Superoxide Ion
While oxygen typically forms two covalent bonds, there are exceptions, particularly when considering specific ionic species like the superoxide ion (O₂⁻). In this ion, oxygen forms only one and a half covalent bonds on average. This unusual bonding arises from the presence of an unpaired electron, resulting in a resonance structure with bond orders between 1 and 2. This creates a weaker bond than the typical two covalent bonds.
Understanding Resonance Structures in Superoxide
The superoxide ion exhibits resonance, a phenomenon where multiple valid Lewis structures can be drawn for a molecule or ion, and the true structure is a hybrid of these contributing structures. In the case of the superoxide ion, one oxygen atom carries a formal charge of -1 while the other carries a formal charge of 0. This distribution of charge leads to a bond order of 1.5.
Oxygen's Role in Biological Systems: A Covalent Bonding Perspective
Oxygen's ability to form covalent bonds plays a vital role in biological systems. The most prominent example is the role of oxygen in respiration. Oxygen's strong tendency to form covalent bonds drives the reactions that release energy from glucose, providing the energy needed for cellular processes. This energy-releasing process involves the formation of new covalent bonds between oxygen and carbon atoms in the glucose molecule.
Importance of Covalent Bonding in Biomolecules
Covalent bonds are crucial in various biomolecules containing oxygen. Consider:
-
Carbohydrates: Oxygen forms numerous covalent bonds within carbohydrate structures, influencing their properties and functions.
-
Lipids: Oxygen is integral to the structure of many lipids, participating in ester linkages that hold together fatty acids and glycerol.
-
Proteins: While not directly involved in the peptide bonds that link amino acids in proteins, oxygen is often found in the side chains of amino acids, influencing their chemical properties and protein folding.
Further Exploration of Oxygen's Bonding Capabilities
The information above covers the fundamental aspects of oxygen's covalent bonding. For a more comprehensive understanding, further exploration can include:
-
Advanced bonding theories: Molecular orbital theory provides a more detailed description of bonding in oxygen and oxygen-containing molecules.
-
Polarity of Oxygen-containing Bonds: The electronegativity difference between oxygen and other atoms often leads to polar covalent bonds, influencing the properties of the resulting molecules.
-
Oxidation States: Oxygen's variable oxidation states, particularly -2, -1, and 0, reflect its versatile bonding behavior and ability to participate in redox reactions.
-
Computational Chemistry: Modern computational tools allow for detailed analysis and prediction of oxygen's bonding behavior in complex systems.
Conclusion: Oxygen's Covalent Bonding: A Cornerstone of Chemistry and Biology
Oxygen's ability to form predominantly two covalent bonds is a fundamental aspect of its chemistry and biology. This bonding behavior is responsible for the properties of countless molecules, from water to complex biomolecules. While exceptions like the superoxide ion exist, the general rule holds true: oxygen's drive to complete its octet by sharing electron pairs dictates its role in shaping the world around us. Understanding this principle provides a crucial foundation for comprehending various chemical and biological processes. Further exploration into the intricacies of oxygen's bonding behavior opens up exciting avenues for research and innovation in numerous scientific fields.
Latest Posts
Latest Posts
-
What Two Regions Make Up All Atoms
Mar 18, 2025
-
Kinetic Energy Of Simple Harmonic Motion
Mar 18, 2025
-
Delta E And Delta H Relationship
Mar 18, 2025
-
Is Boiling A Physical Or Chemical Property
Mar 18, 2025
-
Is Cam Or Kmno4 Better For Alcohols
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Covalent Bonds Does Oxygen Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
