Is Cam Or Kmno4 Better For Alcohols
Muz Play
Mar 18, 2025 · 6 min read
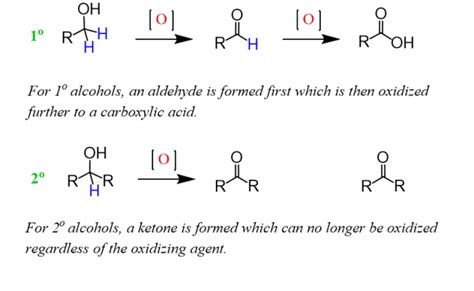
Table of Contents
Is Cam or KMnO4 Better for Oxidizing Alcohols? A Comprehensive Comparison
Choosing the right oxidizing agent for alcohol oxidation is crucial in organic chemistry, impacting yield, selectivity, and the overall efficiency of the synthesis. Two popular choices are chromic acid (often used as Jones' reagent or Collins' reagent) and potassium permanganate (KMnO4). While both can oxidize alcohols, their strengths, weaknesses, and suitability vary considerably depending on the specific alcohol and desired product. This comprehensive analysis dives deep into the comparative performance of chromic acid and potassium permanganate in oxidizing alcohols, helping you make informed decisions in your laboratory work.
Understanding Alcohol Oxidation
Before comparing oxidizing agents, let's briefly review the fundamentals of alcohol oxidation. Alcohols contain a hydroxyl (-OH) group bonded to a carbon atom. Oxidation involves the removal of hydrogen atoms from the alcohol, leading to the formation of a carbonyl group (C=O). The type of carbonyl compound formed depends on the type of alcohol and the reaction conditions.
- Primary Alcohols: These can be oxidized to aldehydes and further to carboxylic acids.
- Secondary Alcohols: These are oxidized to ketones.
- Tertiary Alcohols: These are resistant to oxidation under typical conditions.
Chromic Acid (CrO3) as an Oxidizing Agent
Chromic acid, a powerful oxidizing agent, exists in various forms, most commonly as Jones' reagent (chromic acid in aqueous sulfuric acid) and Collins' reagent (chromic acid complexed with pyridine).
Jones' Oxidation
Jones' oxidation is a vigorous and efficient method for oxidizing primary and secondary alcohols. The strong acidic conditions favor complete oxidation of primary alcohols to carboxylic acids. This makes Jones' reagent unsuitable if you desire aldehydes as your product. Secondary alcohols are cleanly oxidized to ketones.
Advantages of Jones' Oxidation:
- High reactivity: Rapid oxidation, often requiring only a short reaction time.
- High yields: Generally produces high yields of carboxylic acids from primary alcohols and ketones from secondary alcohols.
- Relatively simple procedure: The reaction is straightforward to perform.
Disadvantages of Jones' Oxidation:
- Harsh reaction conditions: The strong acidity can lead to side reactions, particularly with sensitive substrates.
- Toxicity and environmental concerns: Chromic acid is highly toxic and carcinogenic, and the chromium waste disposal presents environmental challenges. Safety precautions are paramount.
- Over-oxidation of primary alcohols: Leads to carboxylic acids, not aldehydes.
Collins' Oxidation
Collins' reagent offers a milder alternative to Jones' oxidation. The use of pyridine helps to moderate the reactivity of chromic acid, making it suitable for oxidizing sensitive alcohols and allowing for better control over the reaction. While it can still oxidize primary alcohols to carboxylic acids under certain conditions, careful control of reaction parameters can often yield aldehydes.
Advantages of Collins' Oxidation:
- Milder conditions: Compared to Jones' oxidation, it's less harsh and more suitable for sensitive substrates.
- Potential for aldehyde synthesis: With careful control, it can be used to produce aldehydes from primary alcohols.
- Improved selectivity: Offers better selectivity compared to Jones' oxidation.
Disadvantages of Collins' Oxidation:
- Requires anhydrous conditions: The reagent is sensitive to moisture, necessitating anhydrous solvents.
- Preparation can be challenging: The preparation of Collins' reagent requires careful handling and specific conditions.
- Toxicity and environmental concerns: Similar to Jones' oxidation, chromium waste disposal remains a significant concern.
Potassium Permanganate (KMnO4) as an Oxidizing Agent
Potassium permanganate (KMnO4) is another powerful oxidizing agent that finds widespread application in organic chemistry. Its oxidation of alcohols often involves basic or neutral conditions.
Advantages of KMnO4 Oxidation:
- Mild to moderate conditions: Depending on the reaction conditions (pH, temperature), the oxidation can be controlled to varying degrees.
- Versatile: Can oxidize various functional groups besides alcohols, including alkenes and alkynes.
- Relatively inexpensive: Compared to chromic acid, KMnO4 is more affordable.
Disadvantages of KMnO4 Oxidation:
- Can be over-oxidative: Under certain conditions, KMnO4 can over-oxidize primary alcohols to carboxylic acids, and it might cleave carbon-carbon bonds in certain substrates.
- Can be slow: The reaction rate is often slower than chromic acid oxidations.
- Manganese dioxide (MnO2) byproduct: The reaction generates manganese dioxide (MnO2), a brown precipitate that needs to be removed from the reaction mixture, potentially complicating workup procedures.
- Reaction conditions crucial: Careful control of pH and temperature is crucial to achieve selective oxidation.
Comparing Cam and KMnO4: A Head-to-Head Analysis
| Feature | Chromic Acid (Cam) | Potassium Permanganate (KMnO4) |
|---|---|---|
| Reactivity | High | Moderate to High |
| Reaction Conditions | Acidic (Jones), Mildly Acidic (Collins) | Basic, Neutral, or Acidic (depending on conditions) |
| Primary Alcohol Oxidation | Carboxylic acid (Jones), Aldehyde (Collins, with control) | Carboxylic acid (often) |
| Secondary Alcohol Oxidation | Ketone | Ketone |
| Tertiary Alcohol Oxidation | No reaction | No reaction |
| Selectivity | Moderate | Moderate to High (depending on conditions) |
| Yields | Generally High | Generally High (dependent on conditions) |
| Toxicity | Highly toxic | Less toxic than chromic acid |
| Cost | Relatively expensive | Relatively inexpensive |
| Waste Disposal | Significant environmental concerns | Less significant environmental concerns (although MnO2 needs handling) |
Choosing the Right Oxidizing Agent
The choice between chromic acid and potassium permanganate depends heavily on the specific alcohol being oxidized, the desired product, and the priorities of the synthesis:
- For high-yield carboxylic acid synthesis from primary alcohols: Jones' oxidation using chromic acid is often the preferred method due to its speed and efficiency. However, safety precautions and waste disposal must be carefully considered.
- For aldehyde synthesis from primary alcohols: Collins' oxidation offers a less harsh alternative, although careful control of reaction conditions is necessary to avoid over-oxidation. Other milder methods, such as Swern oxidation, may be preferred for sensitive substrates.
- For ketone synthesis from secondary alcohols: Both chromic acid and potassium permanganate can be used effectively, with the choice often determined by the availability of reagents and desired reaction conditions. KMnO4 provides a less toxic alternative, albeit often with slower reaction times.
- For oxidation of sensitive substrates: KMnO4 oxidation, under appropriately controlled conditions, might be a gentler option, especially when basic or neutral conditions are employed.
Safety Considerations and Green Chemistry
The use of chromic acid presents significant safety concerns due to its toxicity and the difficulty in managing chromium waste. Potassium permanganate, while less toxic, still requires careful handling. The use of alternative, greener oxidizing agents, such as TEMPO (2,2,6,6-tetramethylpiperidin-1-yl)oxyl) and hypervalent iodine reagents, is becoming increasingly prevalent to reduce the environmental impact of alcohol oxidation. These reagents often provide highly selective oxidations with less toxic byproducts.
Conclusion
Both chromic acid and potassium permanganate are potent oxidizing agents for alcohols, each with its own advantages and disadvantages. The "better" reagent depends entirely on the context of the specific reaction. Factors to consider include the type of alcohol, the desired product, the sensitivity of the substrate, the reaction conditions, and environmental concerns. A careful evaluation of these factors, coupled with a thorough understanding of the reaction mechanisms, will guide you towards the optimal choice for your alcohol oxidation needs. Always prioritize safety and consider greener alternatives wherever possible.
Latest Posts
Latest Posts
-
The Ends Of A Long Bone Are Called The
Mar 18, 2025
-
Electric Potential At A Point Due To A Point Charge
Mar 18, 2025
-
Science Terms That Start With J
Mar 18, 2025
-
What Is The Unit Of Atomic Radius
Mar 18, 2025
-
Which Type Of Hormone Is Lipid Soluble
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Is Cam Or Kmno4 Better For Alcohols . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
