What Is The Unit Of Atomic Radius
Muz Play
Mar 18, 2025 · 5 min read
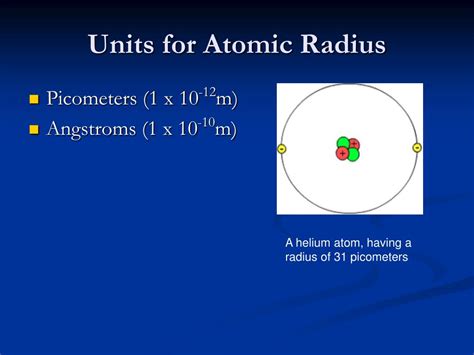
Table of Contents
What is the Unit of Atomic Radius? A Deep Dive into Atomic Structure and Measurement
The seemingly simple question, "What is the unit of atomic radius?" opens a fascinating door into the intricacies of atomic structure and the challenges of measuring something so incredibly small. While a simple answer might be "picometers," understanding the nuances requires exploring the definition of atomic radius, the methods used to determine it, and the inherent limitations of these measurements. This article will delve deep into these aspects, providing a comprehensive understanding of atomic radius and its unit of measurement.
Defining Atomic Radius: Not a Simple Sphere
Before diving into units, let's clarify what we mean by "atomic radius." Unlike a solid ball, an atom doesn't have a sharply defined boundary. Electrons exist in probability clouds, orbitals that describe the likelihood of finding an electron at a particular location. Therefore, defining the "radius" necessitates choosing a specific criterion.
There isn't a single, universally agreed-upon definition of atomic radius. Several approaches exist, each yielding slightly different values:
1. Metallic Radius: For the Metallic Bonds
This applies to metallic elements. It's half the distance between the nuclei of two adjacent atoms in a metallic crystal lattice. This method assumes a close-packed structure, where atoms are arranged as efficiently as possible.
2. Covalent Radius: Sharing is Caring
Used for elements forming covalent bonds (sharing electrons), the covalent radius is half the distance between the nuclei of two identical atoms bonded together. It effectively measures the extent of the electron cloud shared between the atoms.
3. Van der Waals Radius: Weak Interactions, Big Radius
This refers to half the distance between the nuclei of two identical atoms that are not bonded, but are close enough to experience weak Van der Waals forces. This radius is generally larger than metallic or covalent radii due to the weaker interaction. It reflects the outermost extent of the electron cloud's influence.
Why the Variations?
The different methods produce varying results because the electron cloud's extent is influenced by factors like:
- Bonding type: The nature of the bond (metallic, covalent, or Van der Waals) significantly impacts the interatomic distance.
- Oxidation state: The charge on an atom (ion) influences the size of its electron cloud. Ions will have different atomic radii compared to neutral atoms.
- Coordination number: In crystalline structures, the number of neighboring atoms affects the interatomic distances.
The Unit: Picometers (pm)
Despite the variations in defining atomic radius, the standard unit of measurement is the picometer (pm). A picometer is one trillionth of a meter (1 pm = 10<sup>-12</sup> m). This incredibly small unit reflects the minuscule size of atoms. While Angstroms (Å), equal to 10<sup>-10</sup> m or 0.1 nm, were historically common, picometers are now preferred in scientific literature for better consistency within the SI system.
Measuring the Unmeasurable: Techniques and Challenges
Determining atomic radii is not a straightforward process. It requires sophisticated techniques that indirectly infer the atomic dimensions. Some key methods include:
1. X-ray Crystallography: Peering into Crystals
This powerful technique utilizes X-ray diffraction patterns from crystalline solids. By analyzing the diffraction pattern, scientists can deduce the arrangement of atoms and the distances between them within the crystal lattice. This data is crucial for calculating metallic radii and providing insights into covalent radii in crystalline compounds.
Challenges: X-ray crystallography relies on the sample being crystalline and of sufficient quality. Amorphous materials or those with defects can complicate the analysis.
2. Electron Diffraction: A Subatomic Probe
Similar to X-ray diffraction, electron diffraction uses beams of electrons to probe the atomic structure of materials. This technique is particularly useful for analyzing the structure of gaseous or liquid samples that aren't easily crystallized.
Challenges: The interpretation of electron diffraction patterns can be complex, requiring advanced computational techniques.
3. Neutron Diffraction: Seeing the Nuclei
Neutron diffraction uses beams of neutrons instead of X-rays or electrons. Neutrons are sensitive to the position of nuclei within a material, allowing for a more precise determination of interatomic distances.
Challenges: Neutron sources are specialized facilities, and the technique is more expensive compared to X-ray or electron diffraction.
4. Spectroscopic Methods: Energy Levels Reveal Size
Various spectroscopic methods, such as atomic absorption spectroscopy, provide information about the energy levels of electrons in atoms. These energy levels are related to the size of the atom, allowing for indirect estimation of atomic radii.
Challenges: These methods often provide less precise measurements of atomic radius than diffraction techniques.
Trends in Atomic Radius Across the Periodic Table
Understanding atomic radii is crucial for predicting chemical and physical properties. There are notable trends across the periodic table:
-
Across a Period (Left to Right): Atomic radius generally decreases as you move from left to right across a period. This is because the effective nuclear charge (the net positive charge experienced by valence electrons) increases, pulling the electrons closer to the nucleus.
-
Down a Group (Top to Bottom): Atomic radius generally increases as you move down a group. This is because additional electron shells are added, increasing the distance between the outermost electrons and the nucleus.
-
Exceptions: There are exceptions to these trends, particularly in the transition metals and the f-block elements, due to complex electronic configurations and shielding effects.
The Significance of Knowing Atomic Radius
Precise knowledge of atomic radii is not merely an academic exercise. It plays a critical role in several fields:
-
Materials Science: Understanding atomic sizes is essential for designing materials with desired properties, predicting crystal structures, and optimizing the performance of catalysts.
-
Chemistry: Atomic radii influence bond lengths, bond angles, and molecular geometry, determining the reactivity and properties of molecules.
-
Nanotechnology: The ability to manipulate matter at the atomic level depends on a precise understanding of atomic dimensions.
-
Physics: Accurate atomic radius data is vital for theoretical models and simulations of atomic and molecular systems.
Conclusion: A Tiny Measurement with Big Implications
The unit of atomic radius, the picometer, encapsulates the immense challenge of measuring something so incredibly small. While the exact value depends on the chosen definition and measurement technique, the picometer remains the standard unit. Understanding the methods used to determine atomic radii and the periodic trends in atomic size is crucial for advancing our knowledge of materials science, chemistry, nanotechnology, and physics. The seemingly simple question of the atomic radius unit opens a vast and fascinating field of study, emphasizing the intricate relationship between fundamental scientific principles and our ability to measure and understand the universe at its most basic level.
Latest Posts
Latest Posts
-
Ode To Billy Joe Lyrics Meaning
Mar 18, 2025
-
Where Does The Light Independent Reaction Take Place
Mar 18, 2025
-
S P D F Blocks On The Periodic Table
Mar 18, 2025
-
Delta G Of A Carbonyl Reduction
Mar 18, 2025
-
Difference Between A Strong And Weak Acid
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Unit Of Atomic Radius . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
