How Many Electrons Are In Phosphorus
Muz Play
Mar 27, 2025 · 5 min read
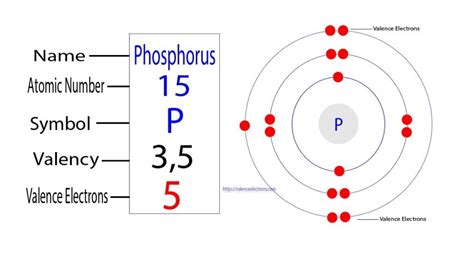
Table of Contents
How Many Electrons Are in Phosphorus? A Deep Dive into Atomic Structure
Determining the number of electrons in an atom of phosphorus might seem like a simple question with a straightforward answer. And it is, to a degree. However, understanding why phosphorus has that specific number of electrons requires a deeper dive into the fascinating world of atomic structure, quantum mechanics, and the periodic table. This exploration will go beyond a simple numerical answer, providing a comprehensive understanding of electron configuration, valence electrons, and the implications of phosphorus's electron count on its chemical behavior.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we tackle phosphorus specifically, let's establish a foundational understanding of atomic structure. Every atom consists of three fundamental subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons defines the element; it's the atomic number.
- Neutrons: Neutrally charged particles also residing in the nucleus. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels. The number of electrons in a neutral atom is equal to the number of protons.
This balance of positive and negative charges ensures the atom is electrically neutral. This fundamental principle is crucial for understanding how many electrons are present in any atom, including phosphorus.
Phosphorus: Its Place on the Periodic Table
Phosphorus (P), atomic number 15, is a nonmetal located in Group 15 (also known as the pnictogens) and Period 3 of the periodic table. Its position on the table provides valuable clues about its electronic structure and chemical properties. The group number indicates the number of valence electrons – the electrons in the outermost shell that participate in chemical bonding. The period number indicates the highest principal energy level occupied by electrons.
The Significance of Atomic Number 15
The atomic number of phosphorus, 15, directly tells us the number of protons in its nucleus. Since a neutral phosphorus atom has an equal number of protons and electrons, a neutral phosphorus atom contains 15 electrons.
Electron Configuration and Orbitals
Electrons don't simply orbit the nucleus randomly. They occupy specific energy levels and sublevels, described by quantum numbers. This arrangement is known as the electron configuration. For phosphorus, the electron configuration is: 1s²2s²2p⁶3s²3p³
Let's break down this notation:
- 1s²: Two electrons in the first energy level (n=1), in the s orbital. The 's' orbital can hold a maximum of two electrons.
- 2s²: Two electrons in the second energy level (n=2), in the s orbital.
- 2p⁶: Six electrons in the second energy level (n=2), in the three p orbitals (px, py, pz). Each p orbital can hold up to two electrons.
- 3s²: Two electrons in the third energy level (n=3), in the s orbital.
- 3p³: Three electrons in the third energy level (n=3), distributed among the three p orbitals. Each p orbital will have one electron before pairing occurs (Hund's rule).
This configuration explains the arrangement of phosphorus's 15 electrons across its energy levels.
Valence Electrons: The Key to Chemical Reactivity
The outermost electrons, those in the highest energy level, are called valence electrons. These electrons are crucial for determining an element's chemical reactivity. They participate in chemical bonds with other atoms, forming molecules and compounds.
In the case of phosphorus, the valence electrons are the five electrons in the 3s and 3p orbitals (3s²3p³). This gives phosphorus a valence of 5, meaning it can potentially form up to five covalent bonds. This high valence explains phosphorus's ability to form a wide variety of compounds.
Isotopes of Phosphorus: Variations in Neutron Number
While the number of electrons in a neutral phosphorus atom is always 15, the number of neutrons can vary. These variations result in different isotopes of phosphorus. Isotopes are atoms of the same element with the same number of protons but different numbers of neutrons. The most common isotope is ³¹P, with 16 neutrons. Other, less common isotopes exist, but all retain 15 protons and, in their neutral state, 15 electrons.
The difference in neutron number affects the atomic mass of the isotope, but it does not alter the chemical properties significantly because chemical properties are primarily determined by the number of electrons.
Phosphorus's Chemical Behavior and its 15 Electrons
The presence of 15 electrons, particularly the five valence electrons, dictates phosphorus's chemical behavior. Its tendency to gain or share electrons to achieve a stable octet (eight electrons in its outermost shell) drives its reactivity.
Phosphorus readily forms covalent bonds, sharing its valence electrons with other atoms. This is evident in various phosphorus compounds, including:
- Phosphine (PH₃): Phosphorus shares three electrons with three hydrogen atoms.
- Phosphorus pentoxide (P₄O₁₀): Phosphorus shares its electrons with oxygen atoms to form a complex structure.
- Phosphoric acid (H₃PO₄): A crucial compound in many biological processes.
Conclusion: The Significance of 15 Electrons in Phosphorus
The seemingly simple answer—15 electrons—to the question of how many electrons are in phosphorus opens a window into the intricate world of atomic structure and chemical bonding. Understanding the electron configuration, valence electrons, and the implications of these factors for phosphorus's chemical behavior highlights the power of the periodic table and the fundamental principles of chemistry. The 15 electrons aren't just a number; they are the key to unlocking phosphorus's unique properties and its essential role in various chemical and biological processes. This exploration extends beyond a simple numerical answer, providing a deeper appreciation for the underlying scientific principles that govern the behavior of matter. The number 15, in the context of phosphorus, represents more than a quantity; it represents a fundamental aspect of its identity and reactivity within the vast chemical landscape. Further study into the behavior of phosphorus and its compounds reveals the complexity and elegance of the natural world, a world built upon the intricate dance of subatomic particles like electrons.
Latest Posts
Latest Posts
-
Why Lipids Are Not Soluble In Water
Mar 30, 2025
-
Adding Integers With The Same Sign
Mar 30, 2025
-
How Do You Divide Fractions With Exponents
Mar 30, 2025
-
N Type Semiconductor Vs P Type Semiconductor
Mar 30, 2025
-
Bipolar Junction Transistor As A Switch
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In Phosphorus . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
