How Many Electrons Are In The Second Energy Level
Muz Play
Mar 15, 2025 · 6 min read
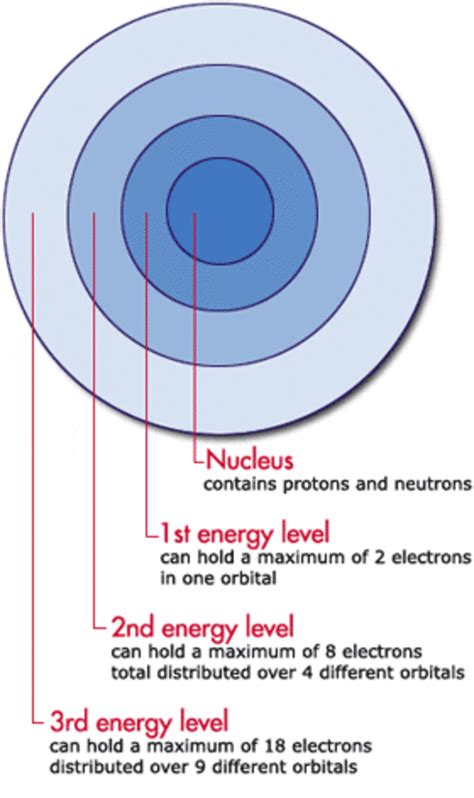
Table of Contents
- How Many Electrons Are In The Second Energy Level
- Table of Contents
- How Many Electrons Are in the Second Energy Level? A Deep Dive into Electron Configuration
- The Quantum World of Electrons: Shells and Subshells
- The Second Energy Level (n=2): A Detailed Examination
- The 2s Subshell: A Spherical Domain
- The 2p Subshell: A More Complex Arrangement
- The Total Electron Capacity of the Second Energy Level
- Electron Configuration and the Second Energy Level
- The Significance of the Second Energy Level in Chemical Bonding
- Ionic Bonds: Electron Transfer
- Covalent Bonds: Electron Sharing
- Beyond the Basics: Hund's Rule and Orbital Filling
- Further Exploration and Advanced Concepts
- Latest Posts
- Latest Posts
- Related Post
How Many Electrons Are in the Second Energy Level? A Deep Dive into Electron Configuration
Understanding electron configuration is fundamental to grasping the behavior of atoms and molecules. This article delves into the specifics of the second energy level, explaining how many electrons it can hold and why, exploring its sublevels, and illustrating its significance in chemical bonding and reactivity.
The Quantum World of Electrons: Shells and Subshells
Before we jump into the second energy level, let's establish some foundational concepts. Electrons don't simply orbit the nucleus like planets around a sun. Instead, they exist in a probabilistic cloud defined by quantum mechanics. We describe their location and energy using the concept of electron shells and subshells.
-
Electron Shells (Principal Energy Levels): These represent the general energy level of an electron. The shell closest to the nucleus (n=1) has the lowest energy, and energy increases as we move further away (n=2, n=3, etc.). The number 'n' is the principal quantum number.
-
Electron Subshells (Sublevels): Within each shell, there are subshells with slightly different energies. These are designated by letters: s, p, d, and f. The s subshell has the lowest energy within a shell, followed by p, d, and f in increasing energy order.
The Second Energy Level (n=2): A Detailed Examination
The second energy level (n=2) is where things start to get interesting. It's significantly more complex than the first energy level (n=1), which only contains an s subshell. The second energy level, however, contains two subshells: the 2s and the 2p.
The 2s Subshell: A Spherical Domain
The 2s subshell is similar in shape to the 1s subshell – a sphere centered on the nucleus. However, it’s at a higher energy level and further from the nucleus. Crucially, like all s subshells, it can hold a maximum of two electrons. This is dictated by the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers.
The 2p Subshell: A More Complex Arrangement
The 2p subshell is significantly different. It consists of three orbitals, each capable of holding two electrons. These orbitals are not spherical like the s orbitals. Instead, they have dumbbell shapes oriented along the x, y, and z axes in three-dimensional space. This arrangement is often visualized as three mutually perpendicular dumbbells. Because each orbital holds two electrons, the 2p subshell can hold a maximum of six electrons (3 orbitals x 2 electrons/orbital).
The Total Electron Capacity of the Second Energy Level
Combining the capacities of the 2s and 2p subshells, the second energy level (n=2) can accommodate a total of eight electrons (2 from 2s + 6 from 2p). This is a crucial number in chemistry, as it explains the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outer shell of eight electrons.
Electron Configuration and the Second Energy Level
The electron configuration of an atom shows how electrons are distributed among its various energy levels and subshells. For example, let's look at some elements and their electron configurations, focusing on the second energy level:
- Lithium (Li): 1s² 2s¹ – Lithium has one electron in the 2s subshell.
- Beryllium (Be): 1s² 2s² – Beryllium has two electrons in the 2s subshell.
- Boron (B): 1s² 2s² 2p¹ – Boron has two electrons in the 2s subshell and one electron in the 2p subshell.
- Carbon (C): 1s² 2s² 2p² – Carbon has two electrons in both the 2s and 2p subshells.
- Nitrogen (N): 1s² 2s² 2p³ – Nitrogen has three electrons in the 2p subshell.
- Oxygen (O): 1s² 2s² 2p⁴ – Oxygen has four electrons in the 2p subshell.
- Fluorine (F): 1s² 2s² 2p⁵ – Fluorine has five electrons in the 2p subshell.
- Neon (Ne): 1s² 2s² 2p⁶ – Neon has a completely filled second energy level with eight electrons.
Neon's stable configuration highlights the significance of the octet rule and the stability associated with a fully filled second energy level.
The Significance of the Second Energy Level in Chemical Bonding
The second energy level's electron capacity plays a vital role in determining an element's chemical properties and how it participates in chemical bonding. Elements with incomplete second energy levels are highly reactive, seeking to either gain, lose, or share electrons to achieve the stable octet configuration of neon. This drive for stability underlies the formation of ionic and covalent bonds.
Ionic Bonds: Electron Transfer
Elements with one or two electrons in their outermost shell (like sodium and magnesium) tend to lose those electrons to achieve a stable electron configuration. This creates positively charged ions. On the other hand, elements with seven electrons in their outermost shell (like chlorine and fluorine) tend to gain an electron to complete their octet, forming negatively charged ions. The electrostatic attraction between oppositely charged ions forms an ionic bond.
Covalent Bonds: Electron Sharing
Elements with four or more electrons in their outermost shell (like carbon, nitrogen, and oxygen) often share electrons with other atoms to achieve a stable octet. This sharing of electrons results in the formation of covalent bonds, the basis of many organic and inorganic molecules.
Beyond the Basics: Hund's Rule and Orbital Filling
The filling of electrons into the subshells follows specific rules. One of these is Hund's rule, which states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration. For example, in nitrogen (2p³), each of the three 2p orbitals gets one electron before any pairing occurs.
Further Exploration and Advanced Concepts
The concepts discussed above provide a solid foundation for understanding the second energy level. Further exploration could delve into:
- Quantum numbers: A more detailed understanding of the four quantum numbers (principal, azimuthal, magnetic, and spin) that define an electron's state.
- Electron-electron repulsion: The effects of electron-electron repulsion on electron configuration and energy levels.
- Shielding effect: How inner electrons shield outer electrons from the full nuclear charge.
- Effective nuclear charge: The net positive charge experienced by an electron after accounting for shielding.
Understanding the second energy level and its electron capacity is essential for comprehending atomic structure, chemical bonding, and the properties of matter. The principles discussed here provide a solid framework for further exploration of these fascinating concepts in chemistry and physics. The eight electrons that this energy level can hold are pivotal in defining the reactivity and bonding behaviors of a vast range of elements. From the simplest ionic compounds to the complex molecules of life, the principles of electron configuration rooted in the second energy level remain fundamental.
Latest Posts
Latest Posts
-
General Chemistry Chapter 1 Practice Problems
Mar 15, 2025
-
Gram Staining Is Classifies As A
Mar 15, 2025
-
Is Density Physical Or Chemical Property
Mar 15, 2025
-
A Process That Does Not Require Oxygen
Mar 15, 2025
-
Is Blood Clotting Positive Or Negative Feedback
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Are In The Second Energy Level . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
