Is Density Physical Or Chemical Property
Muz Play
Mar 15, 2025 · 6 min read
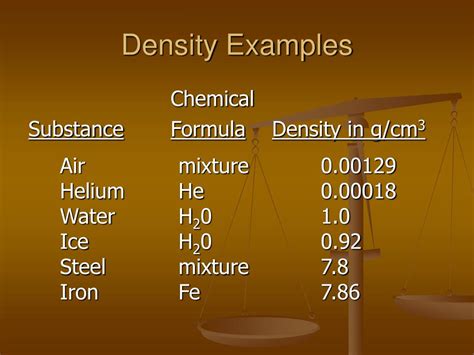
Table of Contents
Is Density a Physical or Chemical Property? A Comprehensive Exploration
Density, a fundamental concept in physics and chemistry, often sparks confusion regarding its classification as a physical or chemical property. Understanding this distinction is crucial for grasping the nature of matter and its behavior. This comprehensive article delves deep into the definition of density, explores its physical nature, differentiates it from chemical properties, and provides examples to solidify understanding. We will also touch upon the practical applications of density and its role in various scientific fields.
Understanding Density: A Definition
Density is defined as the mass of a substance per unit volume. Simply put, it tells us how much matter is packed into a given space. The formula for density is:
Density (ρ) = Mass (m) / Volume (V)
The units for density are typically grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³), although other units can be used depending on the context. It's important to note that density is an intensive property, meaning it doesn't depend on the amount of substance present. A small sample of gold will have the same density as a large gold bar.
Density as a Physical Property: Evidence and Explanation
Density is unequivocally classified as a physical property. A physical property is a characteristic of a substance that can be observed or measured without changing its chemical composition. This is in stark contrast to chemical properties, which describe how a substance reacts or transforms into another substance.
Several key observations support the classification of density as a physical property:
- No chemical change occurs during measurement: Determining the density of a substance involves measuring its mass and volume. These measurements don't alter the substance's chemical makeup. The substance remains the same before, during, and after the density determination.
- Reversible changes: Changes related to density, such as changes in volume due to temperature or pressure, are usually reversible. For instance, heating a substance might increase its volume and thus decrease its density, but cooling it back to its original temperature will restore its original density. This reversibility is a hallmark of physical changes.
- Separation techniques based on density: Many separation techniques, like centrifugation and sedimentation, rely on differences in density. These techniques separate mixtures without altering the chemical composition of the individual components. For instance, separating blood components using centrifugation exploits density differences between the various blood cells and plasma.
Differentiating Density from Chemical Properties
Chemical properties, unlike physical properties, describe a substance's ability to undergo a chemical change. These changes often involve the formation of new substances with different chemical compositions. Examples of chemical properties include flammability, reactivity with acids, and oxidation states.
Here's a table summarizing the key differences between physical and chemical properties, with density highlighted:
| Property Category | Description | Example | Reversibility | Change in Chemical Composition? |
|---|---|---|---|---|
| Physical Property | Observed or measured without changing chemical composition | Density, boiling point, melting point, color, hardness | Often reversible | No |
| Chemical Property | Describes a substance's ability to undergo a chemical change | Flammability, reactivity with acids, oxidation state | Irreversible | Yes |
The crucial distinction is that measuring density doesn't involve a chemical reaction or change the substance's chemical identity. In contrast, observing a chemical property requires inducing a chemical reaction, leading to the formation of a new substance.
Practical Applications of Density
Density's importance extends far beyond theoretical considerations. It finds widespread application in numerous fields:
- Material Science and Engineering: Density is crucial in material selection for various applications. Engineers consider density when designing structures, choosing lightweight materials for aircraft or dense materials for radiation shielding.
- Geology and Geophysics: Density measurements are used to study the Earth's internal structure and composition. Variations in density help geologists interpret seismic data and understand the distribution of different rock layers.
- Medicine and Biology: Density measurements play a vital role in medical diagnostics. Blood density, for example, provides insights into a patient's health. Density gradient centrifugation is a powerful technique used to separate biological molecules and cells.
- Environmental Science: Density measurements help monitor water quality and pollution levels. Changes in water density can indicate the presence of pollutants or changes in temperature.
- Food Science and Technology: Density is an important factor in food processing and quality control. The density of liquids and solids influences various aspects of food processing and packaging.
Factors Affecting Density: Temperature and Pressure
While density is an intensive property, it's not entirely independent of external factors. Temperature and pressure can influence density, but these changes are physical, not chemical.
- Temperature: Increasing the temperature generally causes a substance's volume to expand, thus decreasing its density. This is because higher temperatures provide molecules with more kinetic energy, causing them to move farther apart.
- Pressure: Increasing the pressure generally causes a substance's volume to decrease, thus increasing its density. This is because higher pressure forces molecules closer together.
It's important to note that the effect of temperature and pressure on density varies depending on the state of matter (solid, liquid, gas). Gases are the most compressible and exhibit the most significant density changes with pressure variations.
Density Anomalies: Water's Unique Behavior
Water exhibits a unique density anomaly. Most substances become denser as they cool and solidify. However, water is densest at 4°C (39°F). Below 4°C, the density of water decreases as it approaches the freezing point (0°C). This unusual behavior is due to the hydrogen bonding in water molecules, which causes a unique arrangement of molecules in ice that makes it less dense than liquid water. This anomaly is crucial for aquatic life, preventing bodies of water from freezing solid from the bottom up.
Density and Archimedes' Principle
Archimedes' principle is directly related to density. It states that an object immersed in a fluid experiences an upward buoyant force equal to the weight of the fluid displaced by the object. This principle explains why some objects float while others sink. An object will float if its average density is less than the density of the fluid it's immersed in; otherwise, it will sink. This principle is fundamental in understanding buoyancy and the behavior of objects in liquids and gases.
Conclusion: Density's Firm Place as a Physical Property
In conclusion, density is undeniably a physical property. Its measurement doesn't involve any chemical changes; it's an intensive property, and it's significantly impacted by physical factors like temperature and pressure. The numerous applications of density across diverse scientific and engineering fields highlight its fundamental importance in understanding and manipulating matter. The distinction between physical and chemical properties remains crucial for a thorough comprehension of the behavior of substances and materials. Understanding density allows us to interpret and predict a wide range of phenomena, from the buoyancy of ships to the internal structure of the Earth.
Latest Posts
Latest Posts
-
Difference Between Chemical Reaction And Nuclear Reaction
Mar 17, 2025
-
Which Graph Shows Line Symmetry About The Y Axis
Mar 17, 2025
-
Does Calcium Lose Or Gain Electrons
Mar 17, 2025
-
A Relation Where Every Input Has Exactly One Output
Mar 17, 2025
-
Why Was The Discovery Of Noble Gases A Problem
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Is Density Physical Or Chemical Property . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
