How Many Electrons Do Carbon And Oxygen Share
Muz Play
Apr 05, 2025 · 5 min read
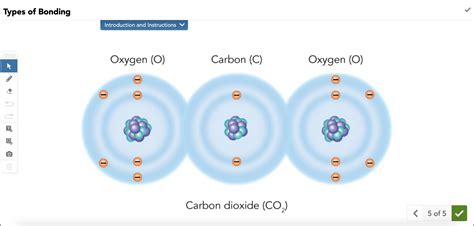
Table of Contents
How Many Electrons Do Carbon and Oxygen Share? Delving into Covalent Bonding
Understanding the number of electrons shared between carbon and oxygen atoms is fundamental to grasping the principles of covalent bonding and the behavior of numerous organic and inorganic molecules. This seemingly simple question opens the door to a deeper exploration of atomic structure, electron configuration, and the formation of stable chemical compounds. This article will comprehensively analyze this question, exploring the underlying concepts and providing examples to solidify your understanding.
Atomic Structure: The Foundation of Sharing
Before diving into the electron sharing between carbon and oxygen, let's revisit the basics of atomic structure. Atoms consist of a nucleus containing protons (positively charged) and neutrons (neutral), surrounded by electrons (negatively charged) in shells or energy levels. The number of protons defines the element's atomic number, while the number of electrons (in a neutral atom) equals the number of protons.
-
Carbon (C): Carbon has an atomic number of 6, meaning it has 6 protons and 6 electrons. Its electron configuration is 1s²2s²2p². This means the first energy level (n=1) has 2 electrons, and the second energy level (n=2) has 4 electrons (2 in the s subshell and 2 in the p subshell). The 4 electrons in the outer shell (valence electrons) are crucial for bonding.
-
Oxygen (O): Oxygen has an atomic number of 8, with 8 protons and 8 electrons. Its electron configuration is 1s²2s²2p⁴. Like carbon, the outer shell (n=2) contains the valence electrons, in this case, 6 (2 in the s subshell and 4 in the p subshell). These 6 valence electrons are vital for oxygen's reactivity.
The Octet Rule: Achieving Stability
Atoms strive for stability, often achieved by having a full outermost electron shell. For many atoms, including carbon and oxygen, this means having 8 electrons in their valence shell—a concept known as the octet rule. This rule is a guideline, and exceptions exist, but it provides a valuable framework for understanding covalent bonding.
Covalent Bonding: Sharing is Caring
Carbon and oxygen achieve stable electron configurations through covalent bonding. Instead of transferring electrons like in ionic bonding, they share electrons to complete their outer shells. Each shared pair of electrons forms a covalent bond, represented by a single line (-) in Lewis structures.
Carbon-Oxygen Bonds: A Deeper Dive
The number of electrons shared between carbon and oxygen varies depending on the specific molecule or functional group. Let's analyze common scenarios:
1. Carbon Monoxide (CO): A Triple Bond
In carbon monoxide (CO), carbon and oxygen share a triple bond. This involves 6 shared electrons: 2 electrons are shared in a single bond, and 4 more electrons are shared in two additional bonds. Each atom effectively gains 3 electrons, completing its octet. This triple bond results in a very strong and stable molecule.
2. Carbon Dioxide (CO₂): Two Double Bonds
Carbon dioxide (CO₂) has a linear structure with carbon in the center. Carbon forms two double bonds with two oxygen atoms. Each double bond consists of 4 shared electrons (2 in a sigma bond and 2 in a pi bond). In total, carbon shares 8 electrons with the two oxygen atoms, completing its octet. Each oxygen atom also shares 4 electrons, completing its octet as well.
3. Methanol (CH₃OH): Single Bond
In methanol (CH₃OH), the oxygen atom is bonded to the carbon atom by a single bond, sharing 2 electrons. Oxygen also forms a single bond with a hydrogen atom. The other two bonds are formed with carbon and hydrogen. The remaining four electrons in the oxygen atom complete its octet. The number of electrons shared between the carbon and the oxygen remains 2.
4. Formaldehyde (CH₂O): Double Bond
Formaldehyde (CH₂O) features a double bond between carbon and oxygen, involving 4 shared electrons. Oxygen and carbon each complete their octet by sharing these four electrons.
5. Other Organic Molecules
The number of shared electrons between carbon and oxygen can vary considerably in other organic molecules, such as ketones, aldehydes, carboxylic acids, and esters. These molecules contain functional groups with carbonyl (C=O) bonds, resulting in double bonds between carbon and oxygen (4 shared electrons). The specific number of shared electrons depends on the overall structure and bonding within the molecule.
Understanding Electron Sharing Through Lewis Structures
Lewis structures are diagrams that show the valence electrons of atoms and how they are shared in covalent bonds. Drawing Lewis structures is a fundamental skill in chemistry, helping visualize the electron sharing between atoms. The steps involved are:
- Count the total valence electrons in the molecule.
- Determine the central atom (usually the least electronegative atom).
- Connect the atoms with single bonds.
- Distribute the remaining electrons to satisfy the octet rule for each atom (except hydrogen, which requires 2 electrons).
- If necessary, form double or triple bonds to complete octets.
Beyond the Octet Rule: Exceptions and Considerations
While the octet rule provides a useful framework, exceptions exist, particularly with elements beyond the second row of the periodic table. Some molecules have an expanded octet, where the central atom has more than 8 electrons in its valence shell. Others have fewer than 8 electrons, forming stable molecules despite incomplete octets. These exceptions showcase the complexity and nuance of chemical bonding.
Conclusion: The Importance of Understanding Electron Sharing
The number of electrons shared between carbon and oxygen varies depending on the molecule or functional group, ranging from 2 electrons in a single bond to 6 electrons in a triple bond, or 4 electrons in a double bond. Understanding this fundamental concept is critical for comprehending the properties, reactivity, and behavior of countless organic and inorganic compounds. By grasping the principles of atomic structure, covalent bonding, and the octet rule, we can gain a much deeper understanding of the chemical world around us. Moreover, the ability to draw and interpret Lewis structures is an essential skill for anyone pursuing a deeper study of chemistry. Mastering this will greatly assist in understanding the intricate dance of electrons that forms the basis of all chemical interactions. Further exploration into molecular orbital theory can provide even deeper insights into the nature of chemical bonds, but the fundamental principles outlined here lay a solid foundation for more advanced study.
Latest Posts
Latest Posts
-
A Chemical Reaction Has Reached Equilibrium When
Apr 05, 2025
-
What Does A Battery Do In An Electrical Circuit
Apr 05, 2025
-
The Electrical Potential Energy Difference Between Two Points
Apr 05, 2025
-
Where Do The Light Independent Reactions Take Place
Apr 05, 2025
-
Place The Steps Of Eukaryotic Dna Replication In Order
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Do Carbon And Oxygen Share . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
