How Many Electrons Does P Have
Muz Play
Mar 21, 2025 · 6 min read
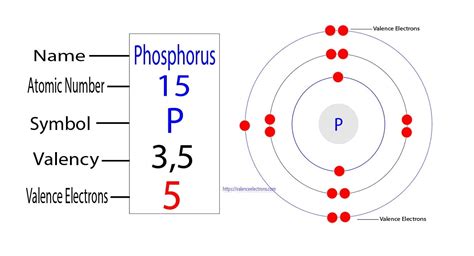
Table of Contents
How Many Electrons Does Phosphorus (P) Have? A Deep Dive into Atomic Structure
Phosphorus (P), a fascinating element crucial for life itself, sparks many questions about its atomic structure. One of the most fundamental is: how many electrons does a phosphorus atom possess? This seemingly simple question opens a door to understanding the intricacies of atomic structure, electron configuration, and the chemical behavior of this nonmetal. This comprehensive guide will not only answer this question but delve into the underlying principles of atomic structure to provide a robust understanding of phosphorus and its properties.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in phosphorus, let's review the basic building blocks of an atom. Every atom consists of three fundamental particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity.
- Neutrons: Neutrally charged particles residing in the nucleus alongside protons. The number of neutrons can vary within an element, leading to isotopes.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons usually equals the number of protons in a neutral atom, ensuring a balanced electrical charge.
Determining the Number of Electrons in Phosphorus
Phosphorus's atomic number is 15. This crucial piece of information tells us that a neutral phosphorus atom has 15 protons in its nucleus. Since a neutral atom has an equal number of protons and electrons, a phosphorus atom possesses 15 electrons.
This seemingly straightforward answer opens the door to a more detailed exploration of where these 15 electrons are located within the atom.
Electron Configuration: Unveiling the Electron Arrangement
Electrons don't randomly orbit the nucleus. They occupy specific energy levels or shells, each capable of holding a maximum number of electrons. These shells are further divided into subshells (s, p, d, and f), each with its own unique shape and capacity for electrons. Understanding electron configuration is key to comprehending an element's chemical properties.
The electron configuration of phosphorus, following the Aufbau principle (filling lower energy levels first), is:
1s² 2s² 2p⁶ 3s² 3p³
Let's break down this configuration:
- 1s²: The first energy level (n=1) contains one subshell, the s subshell, which can hold a maximum of two electrons. Phosphorus has two electrons in this shell.
- 2s²: The second energy level (n=2) also contains an s subshell with two electrons.
- 2p⁶: The second energy level also has a p subshell, which can hold up to six electrons. Phosphorus fills this subshell completely.
- 3s²: The third energy level (n=3) starts with an s subshell containing two electrons.
- 3p³: Finally, the third energy level also contains a p subshell. However, instead of being completely filled, it contains only three electrons. This partially filled p subshell is responsible for many of phosphorus's chemical properties.
Phosphorus's Chemical Behavior and its Electron Configuration
The partially filled 3p subshell is the key to understanding phosphorus's reactivity. Atoms strive for a stable electron configuration, often resembling that of a noble gas (elements in Group 18 with completely filled outer electron shells). Phosphorus can achieve stability by gaining three electrons to achieve a full octet in its outermost shell (3s²3p⁶) or by sharing electrons in covalent bonds.
This explains why phosphorus readily forms compounds with other elements, particularly those that can provide it with the necessary electrons for stability. This propensity for bond formation contributes to phosphorus's importance in various biological and industrial applications.
Isotopes of Phosphorus: Variations in Neutron Count
While the number of electrons in a neutral phosphorus atom is always 15, the number of neutrons can vary, resulting in isotopes. Isotopes are atoms of the same element with the same number of protons but differing numbers of neutrons. The most common isotope of phosphorus is ³¹P, containing 15 protons and 16 neutrons. Other isotopes exist, but ³¹P is by far the most abundant.
It's crucial to remember that the number of neutrons doesn't affect the number of electrons in a neutral atom. The electron count remains consistent regardless of the isotope.
Phosphorus's Role in Biological Systems: The Importance of Electrons
Phosphorus plays a vital role in numerous biological processes. Its electron configuration and ability to form covalent bonds are instrumental to its function:
-
DNA and RNA: Phosphorus is a crucial component of the backbone of DNA and RNA, the genetic materials of all living organisms. The phosphate groups in these molecules link the sugar and base components, creating the characteristic double helix structure of DNA. The electrons shared in the phosphate bonds are essential for maintaining the integrity and functionality of these molecules.
-
ATP (Adenosine Triphosphate): ATP is the primary energy currency of cells. Phosphate bonds in ATP store energy, and the breaking of these bonds releases energy that drives cellular processes. The electron arrangement within these bonds is crucial for energy storage and release.
-
Phospholipids: These are major components of cell membranes, creating a barrier between the cell's interior and its surroundings. The phosphate groups in phospholipids contribute to the membrane's structure and function. Again, the electron interactions within these phosphate groups are key to maintaining the integrity of the membrane.
-
Bones and Teeth: Phosphorus is a vital component of bones and teeth, contributing to their strength and structure. In these structures, phosphorus interacts with calcium to form hydroxyapatite crystals.
In each of these biological roles, the electron interactions and the specific electron configuration of phosphorus are fundamental to its function. The number of electrons, their arrangement within the atom, and their participation in chemical bonds are all essential aspects of phosphorus's biological significance.
Applications of Phosphorus: From Fertilizers to Fire Retardants
Beyond its biological importance, phosphorus finds widespread application in various industrial settings:
-
Fertilizers: Phosphorus is a crucial nutrient for plant growth, making it a key component of many fertilizers. The ability of phosphorus to form stable bonds with other elements makes it an effective nutrient source for plants.
-
Fire Retardants: Phosphorus compounds are used as fire retardants in various materials, slowing the spread of flames. Their effectiveness is related to their ability to interrupt the combustion process by interacting with free radicals.
-
Detergents: Phosphates are used in some detergents, enhancing their cleaning power. However, their use has faced environmental concerns due to eutrophication (excessive nutrient enrichment in water bodies).
-
Metallurgy: Phosphorus plays a role in steelmaking, improving its strength and other properties.
In all these applications, the specific chemical properties dictated by phosphorus's atomic structure and electron configuration are vital.
Conclusion: The Significance of 15 Electrons
The simple answer to "How many electrons does phosphorus have?" is 15. However, this seemingly simple answer opens a door to a vast and fascinating exploration of atomic structure, electron configuration, and the chemical behavior that governs phosphorus's importance in various biological and industrial contexts. Understanding the distribution of these 15 electrons within the atom's energy levels reveals the underlying principles that dictate phosphorus's reactivity, its role in life, and its widespread applications. The significance of these 15 electrons extends far beyond a simple numerical value. They are the fundamental building blocks of a versatile element vital to life and many industrial processes. Understanding their arrangement and the resulting chemical properties is essential for appreciating the multifaceted nature of phosphorus and its significance in the world around us.
Latest Posts
Latest Posts
-
Multiplication And Division Of Mixed Numbers
Mar 22, 2025
-
Periodic Table With Gas Solid Liquid
Mar 22, 2025
-
The Purpose Of Photosynthesis Is To
Mar 22, 2025
-
Examples Of Gas Dissolved In Gas
Mar 22, 2025
-
Is Melting Point An Extensive Property
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does P Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
