How Many Electrons Does Tin Have
Muz Play
Mar 24, 2025 · 5 min read
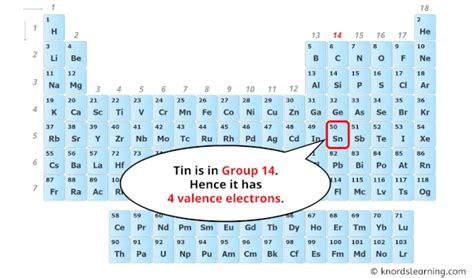
Table of Contents
How Many Electrons Does Tin Have? A Deep Dive into Atomic Structure
Tin, a silvery-white metal known for its malleability and use in various alloys, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly the number of electrons it possesses, is key to comprehending its chemical properties and behavior. This comprehensive guide will delve into the specifics of tin's electron configuration, exploring its implications for its reactivity, bonding capabilities, and overall significance in chemistry and material science.
Understanding Atomic Structure: Protons, Neutrons, and Electrons
Before we pinpoint the number of electrons in tin, let's establish a foundational understanding of atomic structure. An atom, the basic unit of matter, consists of three fundamental subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and its identity on the periodic table.
- Neutrons: Neutral particles (no charge) also residing in the nucleus. They contribute to the atom's mass but not its charge.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. The number of electrons is usually equal to the number of protons in a neutral atom, ensuring a balanced charge.
Tin's Atomic Number and Electron Configuration
Tin (Sn) is found in Group 14 (or IVA) and Period 5 of the periodic table. Its atomic number is 50, which means a neutral tin atom contains 50 protons. Crucially, a neutral atom has an equal number of protons and electrons. Therefore, a neutral tin atom possesses 50 electrons.
However, the story doesn't end there. These 50 electrons are not simply scattered randomly around the nucleus. They occupy specific energy levels, or shells, following the principles of quantum mechanics. This arrangement is described by its electron configuration:
[Kr] 4d¹⁰ 5s² 5p²
Let's break down this configuration:
- [Kr]: This represents the electron configuration of Krypton (Kr), a noble gas with 36 electrons. This notation is a shorthand way of indicating that the inner shells of tin are filled with electrons in the same arrangement as krypton. It simplifies the representation and highlights the valence electrons – those involved in chemical bonding.
- 4d¹⁰: This indicates 10 electrons occupying the 4d subshell. The 'd' subshell can hold up to 10 electrons.
- 5s²: This signifies 2 electrons in the 5s subshell. The 's' subshell can hold a maximum of 2 electrons.
- 5p²: This shows 2 electrons in the 5p subshell. The 'p' subshell can hold up to 6 electrons.
Valence Electrons and Chemical Reactivity
The outermost electrons, those in the highest energy level, are called valence electrons. In tin's case, these are the 2 electrons in the 5s subshell and the 2 electrons in the 5p subshell, for a total of 4 valence electrons. These valence electrons are the key players in determining tin's chemical reactivity and bonding behavior. The tendency of atoms to achieve a stable outer electron shell (like the noble gases) drives their chemical interactions.
Tin can readily lose these 4 valence electrons to form a +4 oxidation state (Sn⁴⁺), forming ionic bonds with electronegative elements like oxygen or chlorine. However, tin can also exhibit a +2 oxidation state (Sn²⁺), losing only the two 5p electrons. This versatility in oxidation states contributes to the diverse range of tin compounds.
Isotopes and Electron Number
While the number of electrons in a neutral tin atom is always 50, it's important to note the existence of isotopes. Isotopes are atoms of the same element with the same number of protons but a different number of neutrons. This means they have different mass numbers but the same number of electrons in their neutral state. Tin has ten naturally occurring isotopes, all with 50 electrons in their neutral form. The difference lies in their nuclear composition (number of neutrons).
Significance of Tin's Electron Configuration in its Applications
The unique electron configuration and subsequent chemical properties of tin explain its extensive use in various applications:
- Alloys: Tin's malleability and ability to form alloys with other metals like copper (bronze) and lead (solder) are crucial in numerous industries, from construction to electronics. The electron configuration dictates its bonding capabilities with these metals.
- Coatings: Tin plating is used to protect other metals from corrosion. The electron configuration influences its reactivity and protective properties.
- Organotin Compounds: Tin forms a wide range of organotin compounds, used as stabilizers in PVC plastics, biocides (antifouling agents), and catalysts. The 4 valence electrons allow for diverse bonding possibilities with carbon and other organic groups.
- Other Applications: Tin is also used in food packaging (tin cans), glass manufacturing, and even certain types of toothpaste. Understanding its atomic structure and electron configuration helps in designing and optimizing these applications.
Tin's Role in the Wider Chemical Landscape
Tin's position in the periodic table, its electron configuration, and consequent chemical behavior play a significant role in our understanding of wider chemical principles. Its ability to exhibit multiple oxidation states provides valuable insights into redox chemistry and its application in various industrial processes. The study of tin's interactions with other elements helps in understanding the complexities of chemical bonding and material science.
Conclusion: A Comprehensive Understanding
The seemingly simple question, "How many electrons does tin have?", leads us on a fascinating journey through the fundamentals of atomic structure, electron configuration, and the impact of these factors on an element's properties and applications. A neutral tin atom invariably has 50 electrons, arranged according to its unique electron configuration ([Kr] 4d¹⁰ 5s² 5p²), which dictates its chemical behavior and its crucial role in a wide array of technological and industrial processes. By understanding this underlying atomic structure, we unlock a deeper appreciation for the material world around us. Further exploration of tin's chemical reactivity, its diverse compounds, and its applications continues to be a vital area of research, constantly expanding our knowledge and paving the way for innovative applications. This detailed analysis emphasizes the importance of understanding fundamental atomic characteristics and their implications in broader scientific contexts. The versatility of tin and its intriguing electron configuration contribute significantly to its relevance in chemistry and material science.
Latest Posts
Latest Posts
-
The Cutaneous Membrane Is Also Known As
Mar 26, 2025
-
Solve The Following System Of Equations Algebraically
Mar 26, 2025
-
Why Are Tertiary Carbocations More Stable
Mar 26, 2025
-
How To Draw An Integral Sign
Mar 26, 2025
-
Which Plane Divides The Body Into Superior And Inferior Sections
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Does Tin Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
