How Many Lone Pairs Does Fluorine Have
Muz Play
Mar 15, 2025 · 5 min read
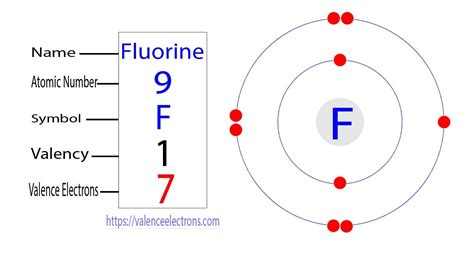
Table of Contents
How Many Lone Pairs Does Fluorine Have? A Deep Dive into Fluorine's Electronic Structure
Fluorine, the most electronegative element on the periodic table, is a fascinating subject for chemical exploration. Understanding its electronic structure, particularly the number of lone pairs, is crucial for grasping its reactivity and properties. This article will delve deep into the electronic configuration of fluorine, explaining how many lone pairs it possesses and why this is significant in its chemical behavior. We'll also explore related concepts like valence electrons, bonding, and the octet rule.
Understanding Electronic Configuration
Before we can determine the number of lone pairs on a fluorine atom, we need to understand its electronic configuration. Fluorine (F) has an atomic number of 9, meaning it has 9 protons and 9 electrons in its neutral state. These electrons are arranged in energy levels or shells around the nucleus.
The electronic configuration of fluorine is 1s²2s²2p⁵. This means:
- 1s²: Two electrons occupy the first energy level (n=1), specifically the 1s orbital.
- 2s²: Two electrons occupy the second energy level (n=2), in the 2s orbital.
- 2p⁵: Five electrons occupy the second energy level in the 2p orbitals. The 2p subshell has three orbitals (2px, 2py, 2pz), each capable of holding two electrons.
Valence Electrons and Chemical Bonding
The outermost shell of an atom, containing electrons involved in chemical bonding, is known as the valence shell. In fluorine, the valence shell is the second energy level (n=2), containing a total of seven valence electrons (2s²2p⁵). These seven valence electrons are crucial for understanding fluorine's reactivity.
Atoms tend to react in ways that achieve a stable electron configuration, often following the octet rule. The octet rule states that atoms tend to gain, lose, or share electrons to achieve a full outermost shell with eight electrons. This stable configuration is similar to that of noble gases, which are highly unreactive.
Lone Pairs and Bonding Pairs
Fluorine, with its seven valence electrons, needs only one more electron to achieve a stable octet. It achieves this by forming a single covalent bond with another atom. This bond involves the sharing of one electron pair between the fluorine atom and another atom.
When we talk about lone pairs, we refer to the pairs of valence electrons that are not involved in bonding. Since fluorine has seven valence electrons and forms one covalent bond (using two electrons), it has three lone pairs of electrons (6 electrons).
Therefore, a fluorine atom has three lone pairs of electrons.
The Significance of Lone Pairs in Fluorine's Chemistry
The presence of these three lone pairs significantly impacts fluorine's chemical behavior:
-
High Electronegativity: Fluorine's high electronegativity stems partly from its strong attraction for electrons, enhanced by the presence of these lone pairs. These lone pairs contribute to the high electron density around the fluorine atom, making it strongly attract electrons from other atoms in a bond.
-
Reactivity: The single unpaired electron readily participates in covalent bond formation, making fluorine highly reactive. It readily forms compounds with most other elements, often with highly exothermic reactions. This reactivity is further influenced by the presence of the three lone pairs.
-
Hydrogen Bonding: The lone pairs on fluorine can participate in hydrogen bonding. Fluorine's high electronegativity and the presence of these lone pairs contribute significantly to the strength of hydrogen bonds formed by fluorine-containing compounds. This is evident in the relatively high boiling points of hydrogen fluoride (HF) compared to other hydrogen halides.
-
Steric Effects: The three lone pairs occupy significant space around the fluorine atom. This creates steric hindrance, influencing the shapes and stability of molecules containing fluorine. In larger molecules, the steric effects of fluorine's lone pairs can affect reactivity and the formation of particular conformations.
Fluorine's Role in Different Compounds
Let's illustrate fluorine's lone pairs with some examples:
-
Hydrogen Fluoride (HF): In HF, the fluorine atom uses one of its valence electrons to form a single covalent bond with the hydrogen atom. The remaining six electrons exist as three lone pairs.
-
Fluoromethane (CH₃F): Similar to HF, the fluorine atom in fluoromethane shares one electron to form a covalent bond with a carbon atom. Its three lone pairs remain unchanged.
-
Difluoromethane (CH₂F₂): Here, the carbon atom is bonded to two fluorine atoms. Each fluorine atom contributes one electron to form a covalent bond, leaving three lone pairs on each fluorine atom.
Beyond the Basics: Advanced Considerations
While the simple model of three lone pairs provides a good understanding of fluorine's basic chemistry, more sophisticated models offer a deeper insight:
-
Molecular Orbital Theory: Molecular orbital theory (MOT) provides a more accurate description of bonding and electron distribution. While it doesn't change the fundamental concept of three lone pairs, MOT reveals a more nuanced picture of the electron density around the fluorine atom and the distribution within the lone pairs themselves.
-
Hybridization: In some molecules, the orbitals involved in bonding and those containing lone pairs might hybridize, affecting the overall electron distribution and molecular geometry.
-
Electron Correlation: Advanced computational methods account for electron correlation effects, which subtly influence the electron density distribution and could slightly modify the description of lone pairs beyond a simplified representation.
Conclusion: The Importance of Lone Pairs in Understanding Fluorine
The seemingly simple question of how many lone pairs fluorine has leads us to a deeper understanding of its electronic structure, reactivity, and the broader principles of chemical bonding. The three lone pairs on a fluorine atom are not just a numerical detail; they are a fundamental aspect of its chemistry, contributing to its high electronegativity, reactivity, hydrogen bonding capabilities, and steric effects. Understanding this fundamental aspect is crucial for comprehending the behavior of fluorine and its numerous compounds across various fields of chemistry and beyond. The interplay between its lone pairs, bonding electrons, and the surrounding atomic environment dictates the properties of countless molecules. This highlights the importance of focusing on the fundamental concepts of electronic structure for a robust understanding of chemical behavior. Further exploration of these concepts using more advanced theoretical models can provide even finer details of fluorine's electronic structure.
Latest Posts
Latest Posts
-
Recall That In Cellular Respiration The Processes Of Glycolysis
Mar 15, 2025
-
How Many Unpaired Electrons Are In Sulfur Atom
Mar 15, 2025
-
Similarities Between The Romanticism And Transcendentalism Movement
Mar 15, 2025
-
Made Up Of Two Glucose Polysaccharides Amylose And Amylopectin
Mar 15, 2025
-
What Moves The Fastest In Tlc
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about How Many Lone Pairs Does Fluorine Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
