How Many P Orbitals Are There
Muz Play
Mar 19, 2025 · 6 min read
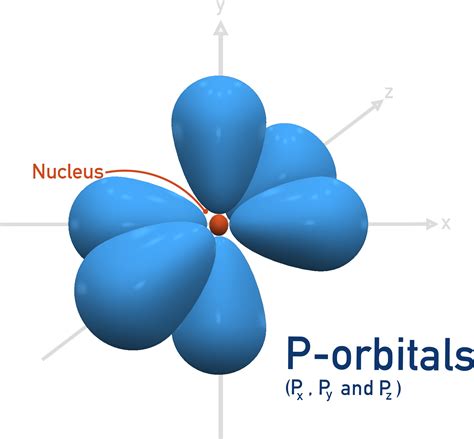
Table of Contents
How Many p Orbitals Are There? A Deep Dive into Atomic Orbitals
Understanding atomic orbitals is fundamental to grasping the behavior of atoms and molecules. While the concept might seem abstract, knowing how many p orbitals exist and their properties is crucial for comprehending chemical bonding, molecular geometry, and the periodic table's structure. This comprehensive guide will explore the world of p orbitals, answering the central question: how many p orbitals are there, and what makes them unique?
Understanding Atomic Orbitals: A Brief Overview
Before delving into the specifics of p orbitals, let's establish a foundational understanding of atomic orbitals. Atomic orbitals are regions of space around an atom's nucleus where there's a high probability of finding an electron. They're not physical boundaries, but rather mathematical descriptions of electron behavior, derived from the solutions to the Schrödinger equation. This equation describes the quantum mechanical behavior of electrons within an atom.
Each orbital is characterized by a set of quantum numbers:
-
Principal Quantum Number (n): This number determines the energy level of the electron and the size of the orbital. It can be any positive integer (1, 2, 3, ...). Higher values of 'n' indicate higher energy levels and larger orbitals.
-
Azimuthal Quantum Number (l): This number defines the shape of the orbital and its angular momentum. It can range from 0 to (n-1). Different values of 'l' correspond to different subshells:
- l = 0: s orbital (spherical)
- l = 1: p orbital (dumbbell-shaped)
- l = 2: d orbital (more complex shapes)
- l = 3: f orbital (even more complex shapes)
-
Magnetic Quantum Number (ml): This number specifies the orientation of the orbital in space. It can take values from -l to +l, including 0. This means that for a given subshell (l), there are (2l + 1) orbitals.
-
Spin Quantum Number (ms): This describes the intrinsic angular momentum (spin) of the electron, which can be either +1/2 or -1/2. This means that each orbital can hold a maximum of two electrons with opposite spins (Pauli Exclusion Principle).
The p Orbitals: Shape, Orientation, and Degeneracy
Now, let's focus on the p orbitals. These are characterized by an azimuthal quantum number (l) of 1. This leads to three possible values for the magnetic quantum number (ml): -1, 0, and +1. This directly answers our main question:
There are three p orbitals in each principal energy level (n ≥ 2).
These three p orbitals are often designated as px, py, and pz, reflecting their orientation in three-dimensional space:
-
px: This orbital lies along the x-axis, with one lobe extending along the positive x-axis and the other along the negative x-axis. A nodal plane (a region of zero electron density) exists in the yz-plane.
-
py: Similar to px, this orbital lies along the y-axis, with a nodal plane in the xz-plane.
-
pz: This orbital lies along the z-axis, with a nodal plane in the xy-plane.
It's important to note that in the absence of an external magnetic field, these three p orbitals have the same energy level. This is known as degeneracy. The presence of an external field can lift this degeneracy, splitting the energy levels slightly.
Visualizing p Orbitals: Beyond Simple Diagrams
While simple dumbbell-shaped diagrams provide a basic understanding, the true nature of p orbitals is more complex. They are three-dimensional probability distributions, and their shape is often misleadingly simplified in introductory chemistry texts. The probability of finding an electron is highest within the lobes, gradually decreasing as you move away from the nucleus. The probability never reaches zero, even at large distances, although it becomes extremely small.
The representation of p orbitals often simplifies the complexity. A more accurate visualization would involve a three-dimensional probability density cloud, showing the likelihood of finding an electron at various points in space. This cloud would be more diffuse than the simple dumbbell model suggests.
The Role of p Orbitals in Chemical Bonding
P orbitals play a crucial role in chemical bonding, particularly in covalent bonds. The overlapping of p orbitals from different atoms forms pi (π) bonds, which are weaker than sigma (σ) bonds formed by the overlap of s orbitals or hybrid orbitals. The shape and orientation of p orbitals significantly influence the geometry of molecules. For example, the double bond in ethene (C₂H₄) involves the overlap of sp² hybrid orbitals (a mixture of s and p orbitals) to form a sigma bond and the sideways overlap of unhybridized p orbitals to form a pi bond.
The number of p electrons an atom possesses greatly influences its reactivity and bonding properties. Elements in the p-block of the periodic table (groups 13-18) have valence electrons in their p orbitals, leading to diverse chemical behaviors. Their reactivity is often determined by the number of unpaired electrons in their p orbitals.
P Orbitals and the Periodic Table
The arrangement of elements in the periodic table is directly related to their electronic configurations, including the filling of p orbitals. The p-block elements (groups 13-18) are characterized by the progressive filling of their p orbitals. The periods (rows) reflect the filling of principal energy levels, with each period containing elements filling the s and p orbitals of that energy level.
For example, the second period (Li to Ne) involves filling the 2s and 2p orbitals. The third period (Na to Ar) fills the 3s and 3p orbitals, and so on. The properties of these elements are largely determined by the number of electrons in their outermost p orbitals. This systematic filling of the p orbitals underlies the periodic trends observed in the properties of elements.
Beyond the Basics: Advanced Concepts
The description above provides a foundational understanding. However, a more in-depth examination reveals further intricacies:
-
Hybridization: In many molecules, p orbitals combine with s and/or other p orbitals to form hybrid orbitals. These hybrid orbitals have different shapes and orientations, leading to varied bonding geometries. Examples include sp, sp², and sp³ hybridization.
-
Molecular Orbital Theory: This theory provides a more sophisticated description of bonding, considering the interaction of atomic orbitals to form molecular orbitals that encompass the entire molecule.
-
Spectroscopy: Various spectroscopic techniques, such as UV-Vis spectroscopy and photoelectron spectroscopy, provide experimental evidence for the existence and properties of p orbitals. These techniques probe the electronic transitions between different energy levels, including those involving p orbitals.
Conclusion: The Significance of Understanding p Orbitals
Understanding the number and properties of p orbitals is essential for a comprehensive understanding of chemistry. From the simple representation of their dumbbell shape to the complex roles they play in chemical bonding and molecular geometry, p orbitals are fundamental building blocks of atomic and molecular structure. Their influence extends throughout the periodic table, shaping the properties and reactivity of a vast array of elements and compounds. This detailed exploration provides a strong base for further studies in chemistry and related fields. The insights provided will aid in comprehending more complex chemical concepts and allow for a deeper appreciation of the fundamental principles governing the behavior of matter. The precise knowledge of the number of p orbitals and their influence on atomic structure is a key cornerstone of our current understanding of chemistry and provides a springboard to further explore the fascinating world of quantum mechanics in chemical systems.
Latest Posts
Latest Posts
-
The Fluid Filled Area Within The Chloroplast Is Called The
Mar 19, 2025
-
How To Find First Term Of Arithmetic Sequence
Mar 19, 2025
-
Nursing Interventions For Patients With Schizophrenia
Mar 19, 2025
-
Three Important Parts Of Microscope Care
Mar 19, 2025
-
Lock And Key Model Of Enzyme Action
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about How Many P Orbitals Are There . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
