How Many Shells Does Potassium Have
Muz Play
Mar 20, 2025 · 5 min read
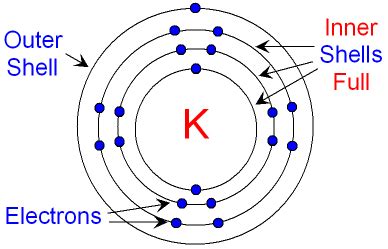
Table of Contents
How Many Shells Does Potassium Have? Exploring Electron Configuration and Atomic Structure
Potassium, a vital element for human health and a key player in various industrial applications, possesses a fascinating atomic structure. Understanding its electron configuration, particularly the number of electron shells, is crucial to grasping its chemical properties and reactivity. This article delves deep into the specifics of potassium's atomic structure, explaining how many electron shells it has, and exploring the implications of this structure on its behavior.
Understanding Electron Shells and Subshells
Before diving into potassium's specific arrangement, let's establish a fundamental understanding of electron shells and subshells. Atoms consist of a nucleus containing protons and neutrons, surrounded by orbiting electrons. These electrons are not randomly distributed but occupy specific energy levels called shells. Each shell can hold a maximum number of electrons, determined by the formula 2n², where 'n' represents the shell number (n=1, 2, 3, etc.).
Within each shell, electrons are further organized into subshells, designated as s, p, d, and f. These subshells have different shapes and energy levels. The s subshell can hold a maximum of 2 electrons, the p subshell 6, the d subshell 10, and the f subshell 14. The filling of these subshells follows the Aufbau principle, which states that electrons fill the lowest energy levels first.
Potassium's Atomic Number and Electron Configuration
Potassium (K) has an atomic number of 19, meaning it has 19 protons in its nucleus and, in its neutral state, 19 electrons orbiting the nucleus. To determine the number of electron shells, we need to understand potassium's electron configuration, which describes how these 19 electrons are distributed among the shells and subshells.
The electron configuration of potassium is 1s²2s²2p⁶3s²3p⁶4s¹. Let's break this down:
- 1s²: The first shell (n=1) contains the s subshell, which holds 2 electrons.
- 2s²2p⁶: The second shell (n=2) contains an s subshell with 2 electrons and a p subshell with 6 electrons, totaling 8 electrons in this shell.
- 3s²3p⁶: The third shell (n=3) contains an s subshell with 2 electrons and a p subshell with 6 electrons, totaling 8 electrons in this shell.
- 4s¹: The fourth shell (n=4) contains only the s subshell, with a single electron.
How Many Shells Does Potassium Have? The Answer
Based on the electron configuration, potassium has four electron shells. The first three shells are completely filled, while the fourth shell contains only one electron. This outermost electron is crucial in determining potassium's chemical behavior.
Implications of Potassium's Electron Configuration
The fact that potassium has four electron shells and a single electron in its outermost shell significantly impacts its properties:
-
Reactivity: The single electron in the outermost shell is relatively loosely held and easily lost. This makes potassium highly reactive, readily losing this electron to form a +1 ion (K⁺). This tendency to lose an electron is characteristic of alkali metals, the group to which potassium belongs.
-
Ionic Bonding: Potassium's tendency to lose an electron allows it to form ionic bonds with other atoms, particularly non-metals that readily gain electrons. This ionic bonding is responsible for the formation of many potassium compounds, such as potassium chloride (KCl) and potassium hydroxide (KOH).
-
Metallic Properties: Potassium exhibits characteristic metallic properties, such as high electrical and thermal conductivity. This is due to the delocalized nature of its valence electrons, which can move freely throughout the metal lattice.
-
Biological Importance: Potassium's reactivity and ionic nature are vital to its biological roles. It plays a critical role in maintaining fluid balance, nerve impulse transmission, and muscle contraction in living organisms. Its presence in cells is crucial for regulating various physiological processes.
Comparing Potassium's Shell Structure to Other Elements
Comparing potassium's electron configuration to other elements helps illustrate the trends in periodic properties. For example:
-
Sodium (Na): Sodium, located directly above potassium in the periodic table, has an electron configuration of 1s²2s²2p⁶3s¹. It has three electron shells. The similarity in their outermost shell configurations explains the similarities in their chemical reactivity.
-
Rubidium (Rb): Rubidium, located below potassium in the periodic table, has an electron configuration of 1s²2s²2p⁶3s²3p⁶4s²3d¹⁰4p⁶5s¹. It has five electron shells. This reflects the increasing number of shells as we move down a group in the periodic table.
Advanced Concepts and Further Exploration
The simple model of electron shells and subshells provides a good understanding of potassium's atomic structure, but more sophisticated models are necessary for a complete description. These models consider:
-
Quantum Mechanics: Quantum mechanical principles describe the behavior of electrons more accurately than the simple shell model. They incorporate concepts like orbitals, wave functions, and quantum numbers.
-
Electron-Electron Interactions: The interactions between electrons in the same shell or subshell influence their energy levels and distribution. These interactions are more complex than what is depicted in the simple shell model.
-
Spectroscopy: Spectroscopic techniques provide experimental evidence supporting our understanding of electron configurations. Analyzing the light emitted or absorbed by potassium atoms can reveal information about the energy levels of its electrons.
Practical Applications and Conclusion
Understanding potassium's electron configuration and the number of its shells is not merely an academic exercise. It has practical implications in various fields:
-
Agriculture: Potassium is an essential nutrient for plant growth and is commonly used in fertilizers. Understanding its chemical behavior is crucial for optimizing fertilizer formulations and improving crop yields.
-
Medicine: Potassium plays a crucial role in maintaining human health. Its concentration in blood is carefully monitored and regulated, and imbalances can lead to serious medical conditions.
-
Industry: Potassium compounds are used in various industrial processes, such as the production of soap, glass, and certain types of batteries.
In conclusion, potassium possesses four electron shells, with the outermost shell containing a single electron. This simple fact underpins its chemical reactivity, ionic bonding tendencies, metallic properties, and its significant biological roles. A deeper understanding of its atomic structure, using both simple models and more advanced quantum mechanical principles, is essential for appreciating its diverse applications in various scientific and industrial fields. Further exploration of the intricacies of its electron configuration can lead to a more comprehensive appreciation of this essential element's significance in the world around us.
Latest Posts
Latest Posts
-
Metals Usually Form What Type Of Ions
Mar 21, 2025
-
Is Melting Point Physical Or Chemical
Mar 21, 2025
-
Are Oxidation Numbers The Same As Charges
Mar 21, 2025
-
Manifest And Latent Functions Of Education
Mar 21, 2025
-
Issuance Of Common Stock Journal Entry
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about How Many Shells Does Potassium Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
