Metals Usually Form What Type Of Ions
Muz Play
Mar 21, 2025 · 6 min read
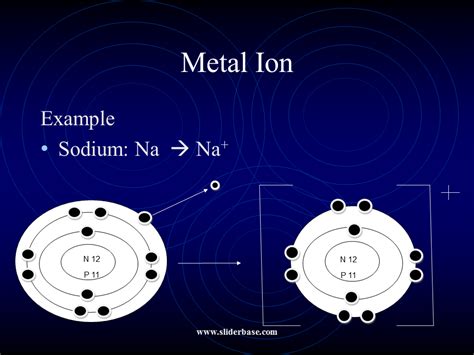
Table of Contents
Metals Usually Form What Type of Ions? A Deep Dive into Cation Formation
Metals are fundamental building blocks of our world, forming the basis of countless materials and technologies. Understanding their behavior, particularly their tendency to form ions, is crucial in various fields, from materials science and engineering to chemistry and biology. This article explores the fundamental reasons why metals typically form positive ions, also known as cations, delving into the intricacies of electronic configuration, ionization energy, and the periodic trends that govern this behavior.
The Octet Rule and Electronic Stability
The driving force behind ion formation in metals is the pursuit of electronic stability. Atoms strive to achieve a stable electron configuration, often resembling that of a noble gas. This is encapsulated by the octet rule, which states that atoms tend to gain, lose, or share electrons to achieve a full outermost electron shell (valence shell) containing eight electrons. While the octet rule isn't universally applicable, it serves as a useful guideline for understanding the behavior of many elements, especially those in the s and p blocks of the periodic table.
Metals, characterized by their low ionization energies and relatively few electrons in their valence shells, find it energetically favorable to lose electrons rather than gain them. This loss of electrons leads to the formation of positively charged ions, or cations. By losing electrons, metal atoms achieve a stable electron configuration, often resembling the noble gas preceding them in the periodic table.
Example: Sodium (Na)
Consider sodium (Na), an alkali metal with an atomic number of 11. Its electronic configuration is 1s²2s²2p⁶3s¹. Sodium has one valence electron in its outermost 3s orbital. Losing this single electron results in a sodium ion (Na⁺) with the electronic configuration 1s²2s²2p⁶, which is identical to that of neon (Ne), a noble gas. This stable configuration makes the ionization process energetically favorable for sodium.
Ionization Energy and its Significance
Ionization energy is the minimum energy required to remove an electron from a neutral gaseous atom or ion. Metals generally have low ionization energies compared to nonmetals. This low ionization energy is a direct consequence of the relatively weak attraction between the nucleus and the valence electrons in metals. The valence electrons in metals are further away from the nucleus and are shielded by inner electrons, reducing the effective nuclear charge experienced by these outermost electrons. Consequently, less energy is needed to remove them.
The successive ionization energies, i.e., the energy required to remove subsequent electrons, generally increase. This is because each subsequent electron is removed from an increasingly positive ion, leading to a stronger electrostatic attraction between the nucleus and the remaining electrons. However, the differences in ionization energies between successive removals for metals are often relatively smaller compared to nonmetals, indicating a relatively easier removal of multiple valence electrons in metals.
Periodic Trends and Cation Formation
The periodic table provides a systematic framework for understanding the trends in ionization energy and, consequently, cation formation. Several key trends are observed:
-
Across a period (left to right): Ionization energy generally increases as we move from left to right across a period. This is due to the increase in effective nuclear charge and a decrease in atomic radius. The increased nuclear charge pulls the valence electrons more tightly, making them harder to remove.
-
Down a group (top to bottom): Ionization energy generally decreases as we move down a group. This is primarily because of the increase in atomic radius. The valence electrons are further from the nucleus and are shielded by more inner electrons, leading to weaker attraction and easier removal.
These trends explain why alkali metals (Group 1) readily form +1 cations, alkaline earth metals (Group 2) readily form +2 cations, and so on. The ease of electron removal directly correlates with their position in the periodic table.
Types of Cations Formed by Metals
The charge on a cation formed by a metal is determined by the number of valence electrons it possesses. Common examples include:
-
+1 cations: Alkali metals (Li⁺, Na⁺, K⁺, Rb⁺, Cs⁺)
-
+2 cations: Alkaline earth metals (Be²⁺, Mg²⁺, Ca²⁺, Sr²⁺, Ba²⁺)
-
Variable charge cations: Transition metals and post-transition metals can form cations with variable charges, depending on the specific conditions and the element involved. For example, iron (Fe) can form Fe²⁺ and Fe³⁺ ions. This variable charge is a result of the complex interplay between the d-electrons and the nuclear charge, enabling multiple oxidation states. The specific cation formed often depends on the reaction conditions and the other reacting species.
-
Higher charge cations: Some metals, particularly those in higher groups, can form cations with higher charges (e.g., Al³⁺, Sn⁴⁺).
Factors influencing cation formation beyond ionization energy
While ionization energy is a key factor, several other factors also influence cation formation:
-
Electronegativity: The electronegativity of the metal and the nonmetal involved in a reaction influences the extent of electron transfer and the stability of the resulting ionic compound.
-
Lattice energy: The lattice energy of the resulting ionic compound contributes to the overall stability of the cation formation. A high lattice energy (strong electrostatic attraction between the cation and anion) favors the formation of the ionic compound.
-
Reaction conditions: The reaction conditions, such as temperature, pressure, and the presence of other reactants, can also influence which cation is formed, especially for metals with multiple oxidation states.
Applications and Importance of Understanding Cation Formation
The knowledge of how metals form cations is crucial in numerous applications:
-
Materials science: Understanding cation formation is crucial in designing alloys and materials with specific properties. The characteristics of an alloy are significantly influenced by the type and charge of cations present.
-
Electrochemistry: Cation formation is fundamental to understanding electrochemical processes like corrosion, batteries, and fuel cells. The movement of cations is essential to the functioning of these technologies.
-
Catalysis: Many metal cations act as catalysts in various chemical reactions. Their ability to accept or donate electrons facilitates the reaction pathways.
-
Biology: Metal cations play vital roles in biological systems. For instance, iron (Fe²⁺ and Fe³⁺) in hemoglobin, magnesium (Mg²⁺) in chlorophyll, and calcium (Ca²⁺) in bone structure are essential for life.
Conclusion
In summary, metals typically form positive ions (cations) because of their low ionization energies and the drive to achieve electronic stability. This behavior is governed by the octet rule, periodic trends in ionization energy, and other factors like electronegativity and lattice energy. Understanding the intricacies of cation formation is critical in diverse fields, highlighting the fundamental importance of metals and their ionic behavior in shaping our world. Further exploration into the specifics of individual metals and their reactions is encouraged for a more complete understanding of this fascinating aspect of chemistry.
Latest Posts
Latest Posts
-
What Is A Metaparadigm Of Nursing
Mar 27, 2025
-
Song Lyrics Ode To Billy Joe
Mar 27, 2025
-
What Happens During The Reduction Stage Of The Calvin Cycle
Mar 27, 2025
-
Is Solid To Liquid Endothermic Or Exothermic
Mar 27, 2025
-
What Does A Negative Enthalpy Mean
Mar 27, 2025
Related Post
Thank you for visiting our website which covers about Metals Usually Form What Type Of Ions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
